Having observed
the fringing reefs found near our home on
Mactan Island since 2004, We have become much more aware of not only how
the various habitats work in conjunction with each other, but also what
threatens
the balance that each of them brings to the coral reef equation. By
taking apart that equation, We hope to show how we can recreate the
reef's
solutions to functionality and apply them to reef aquariums, thus
creating more realistic, and
functional coral reefs within the glass boxes that we like to call our
reef aquariums. This series of articles will examine a Philippine
fringing reef's habitats and how we can apply each of those habitats to
our reef aquarium systems with the intention of creating a more
holistic and functional captive ecosystem that provides multiple
habitats each with their own unique populations that in combination
will provide the means to further the hobby towards establishing
aquarium systems that more accurately represent the meaning of the word
"reef."
Part
Three :
See the Weeds
Previously in this
article series we explored the shoreline and a seagrass meadow.
We now step a bit further out from shore and enter the realm of
the benthic macroalgae. This article will examine yet
another complex marine habitat comprised of macroalgae
(seaweeds),
calcium carbonate rock rubble and sediments creating
an
ecosystem that rivals the seagrass meadows in form and function.
The Fringing
Reef's Macroalgae
The Benthic Macroalgae Habitat
The macroalgae have their greatest biomass and species diversity
within
the
protected reef flats in areas that average 2 meters in depth covering
the majority of the substrates which is comprised of ancient
porous limestone bedrock and calcium carbonate sediment that
averages a depth of 40cm with near complete
surface coverage of
the sediments by porous limestone rock fragments and calcium carbonate
coral rubble. There is great variation in the fragment sizes ranging
from 6cm² up to 100cm².
In contrast to the stable growth patterns of the nearby seagrass
community, the macroalgae grow rapidly and may go through a series of
growth and decline within a single season. With numerous
species
and their variations in the type and amount of nutrients required, the
macroalgae canopy can change dramatically when responding to local
seasonal and eutrophic conditions. What may appear to us as a simple change
in
the weather has a large impact on the nutrient dynamics and
productivity of the macroalgae reef flats through disturbances and nutrient inputs.
Aerial view of
Mactan Island's exposed reef flats
Weather Patterns
- A
seasonal nature.
With two very distinct seasons, a dry cloudless period that runs from
November to May and a wet cloudy monsoonal period that runs from June
to October, the variable effects upon shallow water habitats can be
profound.
The dry season
and its clear sunny days bring
surface water temperatures to their highest yearly average and
available dissolved nutrients to their lowest concentrations.
With the lack of rainfall providing terrestrial runoff and
very
little disturbance during the storm free dry season, the macroalgae
canopy will decline in those species that require consistently higher
levels of dissolved nutrients (i.e. Chaetomorpha spp.).
The wet season and its cloudy nature brings surface water temperatures
down to the lowest yearly average while washing nutrient loaded runoff
into the ocean. Frequent storm driven waves suspends
organic
matter, shifts sediments and sets epiphytic macroalgae adrift. In
particularly heavy rain falls, the local salinity level may become near
brackish having a large impact on the fauna diversity and population
levels, temporarily altering the nutrient dynamics of the entire region.
- Life in the slow lane.
With
the force that water can bring upon a surface area, it is not
surprising that water velocity rates have very profound effects on local
species determining their distribution, community structure, nutrient
dynamics, availability of light and their morphology.
In the
protected reef flats, tidal flows are the primary source of water velocity over
and through the macroalgae habitat on a daily basis while wind driven
wave action is a relatively rare occurance acting as a short lived
disturbance that the macroalgae recover from and in fact may actually
benefit from, as viable algal fragments can be torn away allowing the
dispersal of the species within and out of the local area.
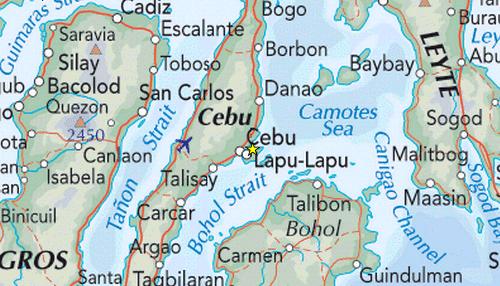
The velocity of tidal currents are defined by local geography
determining the direction and speed at which the tides enter and exit
the reef flats. For the Camotes Sea the surrounding islands form
relatively narrow straits and channels with tidal flows
running parallel to the majority of the coast lines.
For the macroalgae communities located in the shallow reef flats,
the parallel direction of the tidal flows (as shown below)
encounter obstacles that reduce the force of the flows. The
primary force reduction occurs when the flow is directed upwards as it
enters much shallower water depths and strikes the many large boulders
and coral outcroppings creating turbulence within the flow. Once
past the coral reef and its barriers, the reduced flow enters the
deeper edge of the reef flat and its surrounding band of
Sargassum kelp
that through the use of its gas bladders extends its large, wide blades
upwards to the surface creating a living wall for the water flow to run
up against, further reducing the flow. Having moved past the kelp
bed, the flow enters the very shallow areas of the reef flat having
been greatly reduced in velocity only to be further reduced by the
expansive fields of macroalgae before entering the seagrass meadows at
a near standstill. What little velocity that remains is quickly
reduced by the seagrasses leaving the shoreline at near stagnation were
it not for bulk water movement.
The relatively slow water velocities may explain the prevalence of
Phaeophyta species as this algal division has been shown to reproduce at much higher rates in calm conditions in comparison to the
Chlorophyta which release more gametes in higher water velocities
(Gordon 2004).

Having observed the daily, weekly and monthly tidal rhythms for a
number of years now it became apparent that the tides follow a biweekly
cycle between rapid differences in the highs and lows and a much
gentler, expanded time difference between the high and low tides. During the
weeks that the tides are at their highest and lowest, it is by the
sheer bulk mass of 1.5 meters of water depth coming into and draining
out of the reef flats that the velocity blocking structures are
overcome, allowing the exchange of water to occur up to and including
the shallow shoreline areas. However, the bulk movement of the
water can only be described as being
gentle in comparison to the velocities that the fringing
coral reefs are subjected to. These weekly tidal differences also
determine when we go scuba diving on the fringing reefs as we
are
no swimming match against the stronger biweekly tides and restrict
ourselves to the macroalgae habitat and its gentle waters during these
week long tidal events.
We would be remiss if we did not
mention that large shipping traffic and their short lived, yet large
wakes also appears to have quite a large affect upon the reef flats.
We have observed on numerous occasions that a large passing ship
can create the same wave action normally associated with storm activity
during the wet season although of a very short duration.
Having three or four large swells wash over the reef flats quickly
resuspends
any particulates only to have them settle back down
within a matter of a few hours. With gentle tidal flows, the
suspended particles do not appear to drift far before they settle once
again. Regardless of what may appear to be a slight disturbance, it is
a disturbance and must have an affect on the nutrient dynamics of
both the sediment and the macroalgae and may, or may not have
greater implications for the coral reefs. We feel that this is an
area of concern that warrants further study.
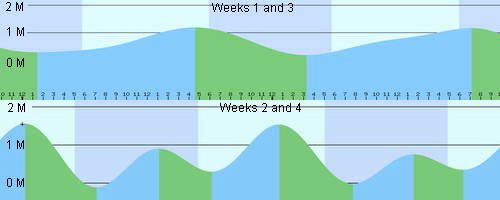 Average monthly tidal movements of Cebu Island, The Philippines
Average monthly tidal movements of Cebu Island, The Philippines
When the conditions are tide-dominated at reduced velocities, the
blades of the macroalgae tend to lean over in one direction for several
hours before changing direction with the tidal changes forming a
relatively sealed environment between the blades of taller macroalgae
and becoming near stagnant within the thick mats of the low growing
species. This slow diffusion of CO² and nutrients through the
macroalgae fields has a strong influence on the community structure of
the reef flats as only those species capable of withstanding long term
limitations will survive. In areas that are wave-dominated, the
environment for the macroalgae is vastly different with much greater
forces being exerted upon them and allowing only those species capable
of withstanding the great mechanical stresses to survive, such as those
species with filamentous and crustouse morphologies. The larger,
frondose species would simply be torn away and driven onto shore.
The
Sediment - Composition and nutrient dynamics

The largest controlling factor for the
colonization and suvivorship of sediment infauna is the sediment
itself, its composition and sizes of material, be it calcium carbonate
or
terrigenous
in source. Sand grain sizes also determines the
nutrient dynamics of the sediment and its suitability for habitation by
infauna, influencing community structure.
In contrast to the very fine grained (mud)
sediment of the seagrass meadows, the macroalgae reef flat's sediment
is
comprised of larger grain sizes attributable to the reef flat
being farther offshore and subject to greater water velocities and storm
driven events that limits
or prevents the permanent settlement of small particulates or sediments associated with seagrass meadows.
 Calcium carbonate sediment and the many remains of animals who once used calcium carbonate to form their structures.
Calcium carbonate sediment and the many remains of animals who once used calcium carbonate to form their structures.
The sediments of reef flats can of course be variable in their
composition but for the majority of the fringing reefs that hold or
have held coral reefs, the sediments are nearly 100% calcium carbonate.
This type of sediment composition plays a large role in
the nutrient dynamics of the sediment by not only adsorbing phosphates
and providing the anoxic conditions for denitrification but can
also contribute elements laid down by reef building organisms
long ago
as the calcium carbonate decomposes and dissolves, returning to the
surrounding water the micronutrients that were once abundantly
accumulated through carbon fixation. Over the long term, organisms
themselves can modify the geochemical evolution of the environment,
however slight that may be
(Khristoforova 1980).
Unlike the seagrasses that gain their nutrients directly out of the
sediments by way of their roots, the macroalgae rely upon dissolved
nutrients within the water making them much more dependent on other
nutrient processes and will take what they can, when they can,
regardless of the amounts being made available to them.
With high cover of the sediments by mat forming algae species and the
resultant loss of water velocity through the algal mats, any
particulates that do accumulate quickly settle between the larger sand
grains providing a nutrient source for the infauna as well as a limited
supply of dissolved organics to the macroalgae. With
ogliotrophic
conditions on the reef flats, the slow growing, mat forming macroalgae
quickly uptake what little nitrogen or phosphorous that the sediment
may release while limiting the influx of dissolved nutrients to the
sediments by performing nitrification and denitrification above the
surface of the sediment, thus partially filling the role that bare
sediments have upon nutrient cycling.
Nearby seagrass meadows play a large role in providing organic
matter through the contribution of their vegetative matter and
detritus when disturbances suspend and distribute such material
throughout the entire reef flat. Such material can fall upon the
macroalgae and through decomposition provide a localized source of
dissolved organics that the macroalgae are able to quickly uptake.
However, the shading of the macroalgae by material such as
seagrass
blades may offset any derived nutritional benefits.
Detritus
accumulation further enriches the near sediment regions of the algal
mats providing another source of organics to both the macroalgae and
the sediments. While storm driven drift macroalgae is often
accused of damaging the seagrass meadows, the same can be said of the
seagrasses by their depositing leaves upon the macroalgae and
having their fine particulates suspended, reducing the clarity of the
water and the passage of light.
Of course the macroalgae also
make their own nutrient contributions to not only their localized area
but to the entire reef flat by their sequestering large amounts of
nutrients and their being set adrift and translocated elsewhere only to
end up as nutrient rich decaying matter themselves if they are unable
to survive the conditions they are transported to, or by being consumed by
any number of herbivores that normally do not frequent the shallow reef
flats.
Bacteria "
Its a dirty job but someone has to do it",
and thankfully the bacteria do their jobs and do so in ways that no
other organism can, using every natural and most man-made compounds on
the planet. In the marine environment they are responsible for
the cycles of nitrogen, oxygen, carbon, sulfur, phosphorous, iron and
many other bioelements that sustain and make possible, life within the
oceans.
Most bacteria may be placed into one of three
groups based on their response to oxygen. Aerobic bacteria thrive
in the presence of oxygen and require it for their continued growth and
existence. Other bacteria are anaerobic, and can not
tolerate oxygen, such as those bacteria which live deep within the
sediments. The third group are the facultative anaerobes, which prefer
growing in the presence of oxygen, but can continue to grow without it.
Further simplified, bacteria can be either heterotrophs or autotrophs. Heterotrophs derive energy from
breaking down complex organic compounds that they must take in from the
environment, this includes saprobic bacteria found in decaying material, as
well as those that rely on fermentation or respiration.
The autotrophs fix carbon dioxide to make their own food source,
this may be fueled by light energy (photoautotrophic), or by oxidation
of nitrogen, sulfur, or other elements (chemoautotrophic). While
chemoautotrophs are uncommon, photoautotrophs are common and quite
diverse. They include the cyanobacteria, green sulfur bacteria, purple
sulfur bacteria, and purple nonsulfur bacteria. The sulfur bacteria are
particularly interesting, as they use hydrogen sulfide as a hydrogen
donor instead of water like most other photosynthetic organisms.
 The various zones can be highly variable in the depths at which they occur.
The various zones can be highly variable in the depths at which they occur. Just as all living organisms, bacteria need to gain energy and nutrition in order to live, grow and
reproduce. However, bacteria are far too small to have a mouth. Instead they
have special channels in their cell walls and cell membranes which allow, and
even assist some molecules to cross. Once the molecules are inside the bacterial cell they
are broken down into their component parts before being rebuilt into the
macromolecules the bacteria needs in order to generate energy or to build and repair itself.
Unfortunately for the bacteria, the surrounding environment is
not always full of free-floating molecules of the correct sort, instead
the molecules may be all bound together. To solve this problem
bacteria have evolved the ability to excrete enzymes out into the
environment around them. These enzymes then "attack" specific tissues
and molecules (proteases attack proteins, cellulases attack cellulose
etc) and break them up into smaller units which other
organisms also find of use and benefit from. Eventually molecules
of a
size that the bacteria can take into itself through the channels
mentioned previously are created.
 1-Pili assist the bacteria in attaching to other cells and surfaces. 2-Ribosomes are microscopic "factories" found in all cells.
1-Pili assist the bacteria in attaching to other cells and surfaces. 2-Ribosomes are microscopic "factories" found in all cells.
3-Cell Envelope is made up of two to three layers. 4-Flagella are hairlike structures that provide a means of
locomotion.
5-Cell Wall gives the cell its shape and protects it from the environment. 6-Chromosome, a single continuous strand of DNA.
7-Plasmids are small, extrachromosomal genetic structures that replicate independently of the chromosome.
Illustration © BiologyCorner. Reprinted with permission. Bacterial metabolism is classified on the basis of three major criteria,
the kind of energy used for growth, the source of carbon, and the electron
donors used for growth. An additional criterion of respiratory microorganisms
are the electron acceptors used for aerobic or anaerobic respiration.
Cellular respiration describes the metabolism reactions and
processes that take place in a cell to obtain biochemical energy from fuel
molecules and the release of the cell's waste products. Energy is released by
the oxidation of fuel molecules and is stored as "high-energy" carriers. The
reactions involved in respiration are catabolic reactions in metabolism.
Fuel molecules commonly used by cells in respiration include glucose,
amino acids and fatty acids, the most common oxidizing agent (electron acceptor) is
molecular oxygen (O²). There are bacteria however, that can respire
using other organic molecules as electron acceptors instead of oxygen. Those
that use oxygen as a final electron acceptor in respiration are described as
aerobic, while those that do not are referred to as anaerobic.
The energy released in respiration is used to synthesize molecules that
act as a chemical storage of this energy. One of the most widely used compounds
in a bacterial cell (and algae) is adenosine triphosphate (ATP) and its stored chemical energy can be
used for many processes requiring energy, including biosynthesis, locomotion or
transportation of molecules across cell membranes. Because of its ubiquitous
nature, ATP is also known as the "universal energy currency", since the amount
of it in a cell indicates how much energy is available for energy-consuming
processes.
Viruses Marine
viruses play a number of roles in the ecology of marine
microbes, primarily acting as predators on other microbes at rates that
equal or exceed losses by grazing. Such vast daily destruction of
microphytobenthos and phytoplankton populations has an large, important impact
on the regulation of nutrient cycles by viral
lysis,
liberating nutrient and trace elements to the microbial loop.
While viral activities may seem to be destructive, it is through
the act of destroying that allows other microbes more bioavailable
material. A study of viral lysis on cultures of phytoplankton
(Gobler 997) showed
that viral lysis released dissolved organic carbon at nearly 160 %
of levels in uninfected control cultures. Calculations based on the
data suggests that lysis of a typical phytoplankton bloom could
increase DOC levels by about 40 mM. This is a large input
of organic carbon that would increase dissolved organic
carbon by as much as 29% over a period of a few days. Their
experiments demonstrated that viral lysis of phytoplankton can result
in both a sudden and large release of dissolved organic carbon, and a
rapid increase in bacterial growth rates and productivity fueled by
viral actions.
 Viral lysis upon phytoplankton and bacteria has a direct impact on the
flux of organic carbon
Viral lysis upon phytoplankton and bacteria has a direct impact on the
flux of organic carbon
and the recycling of nutrients whereas grazing
channels nutrients to the higher trophic levels.
Another important viral impact, and one that can affect global weather
patterns is the viral lysis release of dimethylsulfoniopropionate
(DMSP) from phytoplankton, upon the release of DMSP, free-living
heterotrophic microbes act upon the DMSP and create dimethylsulfide
(DMS) increasing its concentration and forcing greater flux to the
atmosphere where it can affect the formation of cloud cover. Other
processes known to create DMS are the grazing of algal cells by small
planktivores and algal
senescence (Hill 1998).
Also of interest is that in their infectious
attacks the viruses help to maintain the high levels of microbial
genetic diversity as the hosts are known to manipulate their genomes to
evade diseases. Additionally, the viruses also move DNA between the
host cells providing new combinations of genes in their hosts, a
type of sex? This DNA movement is likely the means in which many
microbes gained the ability to photosynthesize as the viruses are known
to transport the genes necessary to do so. This temporary storage
of DNA within the viruses and the movement and mixing of their prey's
genes most likely had and continues to have a profound impact on
the evolution of marine microbes. They may be pathogens, but are most
likely responsible for many evolutionary steps. Not such the bad guys
after all.
Microphytobenthos (Microscopic photosynthetic organisms living in, on or near the sediment)

Within the patchwork of unshaded and so called
unvegetated sediments found throughout the macroalgae habitat live
mixed communities of microscopic
pennate
diatoms, dinoflagellates, and cyanobacteria forming sediment
communities occupying several microhabitats within, on and near the
sediments suggesting that they have multiple functions and are
very important contributors to primary production.
While
not always obvious as to their being present, hence the use of the term
"unvegetated" in many studies when describing what appears to be bare
sediments. The microphytobenthos are there, and in numbers that
usually far exceed that of the phytoplankton in the water column,
although in shallow reef flats the descriptive terms of
phytoplankton and microphytobenthos can be deceiving. In calm
conditions, the microalgae species normally found as phytoplankton can
drop out of the water column and if sufficient light is present,
continue to photosynthesize and become part of the microphytobenthos,
the reverse is also possible when disturbances lift the
microphytobenthos into suspension and they themselves become part of
the phytoplankton.
(MacIntyre 1996).

These functional
autotrophs and
mixotrophs can be found living epiphyticaly on the sand grains and macroalgae while
epipelic
groups of diatoms and dinoflagellates migrate within the sediments
acting as food for sediment infauna, sediment stabilizers that help to
prevent particle suspension and as regulators of nutrient exchange
between the sediments and the water column
(Heil 2004).
As with any photosynthetic organism, the microphytobenthos are oxygen
producers potentially influencing sediment nutrient dynamics in the top
few centimeters which aides nitrification and denitrification.
Being growth responsive to nutrients in the water column they
cause an exchange of dissolved nutrients from the water to the
sediments by carbon fixation and by their being consumed by microinfauna
results in their contributing a percentage of their sequestered
nutrients as detritus into the sediment.
In cases of
eutrophication, the epiphytic microalgae can overwhelm the macroalgae
defenses against such growths and directly compete with the macroalgae
for dissolved nutrients, in extreme cases the epiphytic microalgae can
shade the macroalgae causing a loss of photosynthesis resulting in
the decline of growth and nutrient uptake by the macroalgae, magnifying
the competitive effect in favor of the microalgae.
 Benthic microalgal response after 7 days exposure to nutrient enrichment in microcosms at 50% incident irradiance. nd=no data.
Benthic microalgal response after 7 days exposure to nutrient enrichment in microcosms at 50% incident irradiance. nd=no data.
Response
is given as a stimulation factor, the ratio of the treatment response
relative to control response (dotted line) which received no enrichment.
Data compiled from Heil 2004
Nutrient enrichment studies have shown that microalgae have a slight
preference for a N & P nutrient balance but are quite capable of
sustained growth in unbalanced enrichments, allowing the cells to
persist when larger autotrophs are nutrient limited by one or the other
and only become limited themselves when the levels of both N
& P fall. This is most likely attributable to there being
multiple species in the benthos community, each having its own
limitations allowing for the growth and decline of species yet able to
maintain the communities population albeit a shift in structure and thus its productivity.
However, the biofilm communities have been shown to be remarkably adept
at sequestering and retaining organic carbon and nitrogen through a
variety of biochemical mechanisms. Given the propensity of calcium
carbonate sediments to bind phosphorous, the nitrogen
compounds would first become limiting as there is little nitrogen
held by geochemistry actions, but despite being faced with chronic
nitrogen and carbon limitation, the microphytobenthos optimize carbon
and nitrogen sequestering and retention which tends to minimize any
limitations.
The formation of biofilms on the surface of the sediment can to some
degree prevent movement of the sediment's surface grains through the
excretion of extracellular polymeric substances (EPS) by diatoms, a
considerable part of their photosynthetically fixed carbon (30-73%) is
laid down as EPS that acts as a binding agent upon the grains of the
sediment. EPS consists primarily of glucose, a high energy source
for bacteria and is an important link for the transfer of carbon
within the microbial food web. The diatoms have also been observed
to use their own EPS production as a low nutrient but high energy
source when deprived of light. Storing energy for a rainy day?
In addition to the surface activities and their effects, many of
the free-living cells within the sediments have rhythms of
vertical migration, moving towards the surface when the sediment is
exposed at low tide and descending before it is flooded, doing so may
help the microphytobenthos avoid being suspended by the incoming tides.
Being microscopic, the speed at which the microphytobenthos move is
very low, on the order of 10 to 27mm per hour. During their migrations,
the cells continue to photosynthesize and uptake nutrients as the
distance over which light, nutrient availability and redox potential
vary is equally as small
(MacIntyre 1996).

It
may be tempting to overlook these microscopic producers and
focus solely on the larger macroalgae but given the profound effect
that so many tiny individuals have upon sediment nutrient
dynamics and their large contribution to primary production (up to
50%), they must be taken into account and appreciated when studying any
marine habitat that has exposed sediments and sufficient light
intensity to stimulate photosynthesis. The volumes of quality
food produced by the microphytobenthos forms the basis of many marine
food webs. They are truly a wonder.
Meioinfauna & Pseudomeiobenthos 
Most of the ocean's bottom is covered in sediments, making
the organisms that live within the sediments the largest faunal group
on Earth, sampled areas have led to estimates of infaunal species
numbers ranging from 500,000 up to 10,000,000, most of which have yet
to be described by science
(Snelgrove 1998).
With high productivity provided by microbes and microphytobenthos the
meioinfauna population densities and diversity can be as equally as
large. Productivity studies from around the world show a high
correlation between availability of food sources and diversity.
Population densities are also affected by other factors such as
salinity and temperature but
granulometric
sediment composition has the greatest influence. Numerical analysis of
meiofauna density distributions in different sediment types showed that
Harpacticoids (copepoda) dominated in sediments composed of mainly
coarse grains and by Nematodes in mixed sand and silted sediments.
Meiofaunal grazing can be controlled by primary producers such as
microalgae and when these sources are in short supply, bacteria
represent a readily available food source. Furthermore,
macrofauna may compete for the same food sources and utilize
meiofauna as a food source, exerting another controlling factor on the
meiofauna.
Data compiled from multiple studies from
across the world show similar meiofauna community structures and
densities in both tropical and sub-tropical locations. Sediment
granulometric composition appearing to have the largest affect on all
taxon groups.

Nematodes, by far the most abundant of the meiofauna regardless
of sediment conditions, found in both course grained and fine grained,
silted sediments comprising 22-84% of the meiofauna community
structure. With an estimated one million species and only a few
hundred actually known, there is still a great deal to learn of these
worms. Despite sharing basic morphology, nematodes occupy
numerous roles in sediments, feeding on bacteria, on algae or on
both, they eat detritus and possibly dissolved organic matter while quite a
few are predators, feeding on other nematodes, oligochaetes and
polychaetes. Their diversity in feeding reflects their species
diversity making the number of nematode species in most
habitats much higher than that of any
other group, hence their extremely important roles in sediment nutrient dynamics.

Harpacticoids are found to be restricted to the larger grained
sediments comprising 30-37% of the meiofauna community structure but
only 8-12% in fine grained silted sediments due to the lowered oxygen
content of such sediments. Harpacticoid copepods are known to eat
a variety of foods, including bacteria, algae, and detritus but
seem to prefer diatoms with different copepod species consuming a
preferred diatom size in relation to their own size
(Troch 2006).
Second only to the nematodes in biomass and the transferring
of nutrients garnered from microphytobenthos, the copepods are a
vital component of the benthic food web.

Pseudomeiobenthos while a mixture of juvenile species, they do comprise
8-35% of the meiofauna community with their greatest numbers to be
found in fine grained silty sediments. While the
pseudomeiobenthos also make up a large percentage of the meiofauna,
they do so not by single species population densities but by there
being many species in combination, juvenile Polychaetes being the
most numerous. Bivalves make up the second largest group to be
found.

Ostracods have their greatest density in fine grained silted
sediments comprising up to 10% of the meiofauna community while in
larger, course grained sediments their density level drops down to less
than 1%. One of the most successful crustacean groups
with 8000 living species with very diverse diets. The
majority of the species are found within sediments consuming organic
detritus, algae or other members of the meiofauna. Ostracods are
segmented crustaceans having a head, thorax and abdomen and while
bivalved, they are not clams. Only the head has the full
complement of limbs with five pairs of "hairy" legs and at least one
eye.
With such vast numbers, the meiofauna are a very important food source for
higher trophic levels, particularly for macrofauna, small fish species,
juveniles of larger fish species and other epibenthic predators
, subjecting
them to significant predatory pressure resulting in high levels
(up to 75%) of meiofaunal production being channeled to higher trophic
levels. Polychaetes and Nematodes are the major contributors to such
energy transfer, most likely a result of their large individual
biomass (for Polychaetes) and high abundance (for Nematodes).
However, with high recruitment and reproduction, the population
densities of meiofauna remain at consistent levels
(Danovaro 2007). Macroinfauna 
A
species sampling
(Nacorda 1997) performed
within the sediments of a reef flat offshore of Santiago
Island, The Philippines, encountered 98 taxa from
11 major groups. The majority of which were members of the Annelida.
The Polychaete order Phyllodocida containing 13 families represented
the most abundant order. The arthropods consisted of 20 crustacean taxa
(malacostracans and ostracods) and 2 chelicerate taxa (marine mites and
sea spiders). Gastropods, bivalves, and scaphopods (Dentaliidae) made
up the mollusks. Other groups encountered were the cnidarians
(young octocorals and hydroids) and echinoderms (small ophiuroids,
holothurians, and echinoids), flatworms (Turbellaria), chaetognaths
(the benthic genus Spadella), nemerteans, and sipunculids. Fish larvae
and cephalochordates (Branchiostoma sp.) represented the chordates.
The sampling site as described within the study is very similar in all
aspects to the reef flats here on Mactan Island. Both sites share nearly
identical features per area size, water depth, fauna and infauna, tidal
flows and subjected to the same seasonal
variations.
 Salinity
Salinity had
the largest impact for most groups population levels during
the monsoonal wet season as shown in the population chart
compiled
from Nacorda's study (see figure #1). With increased
rainfall,
the lowered salinity causes a drop in the macroinfauna's population
along with a shift towards diminished diversity among the groups
sampled. It is unclear what affects this temporary decline in
macroinfauna populations has upon the sediment's nutrient dynamics but
one could assume that a drop in population levels of any infaunal group
would reverberate up and down the food web.
Temperature
variation was shown to have no significant impact
(Nacorda 1997) on the
macroinfauna. Between the years 2005 and 2007 I have taken
water
temperature readings on a weekly basis and at the same time of day
(1-2pm). During the dry, cloudless and thus warmer season the
average surface temperature is 32°C (89°F).
The wet,
cloudy and thus cooler season averages a surface temperature of
28°C (82°F).

The mixture of coral rubble fragments within the
carbonate sediment provides macroinfauna stabilizing structures in
which to form extensive burrows. The soft sediments provide habitat for
multitudes of macroinfauna that are capable of creating tube
linings and microinfauna that simply live between the grains of the
sediment.
Macrofaunal structures, as shown above, represent an important
microenvironment within the sediment. Such burrows formed by
bottom-dwelling animals irrigate oxygen deep into the sediment having a
large effect upon microbial communities. With oxygen being the most
favored electron acceptor for bacterial respiration, the lack of oxygen
can have a very negative affect on the microbes, restricting them to
the thin surface boundary within the sediments. With increased
bacterial activity and the respiration of the animals that create the
burrows, a greater amount of CO² is made available to the
macroalgae according to a study (
Kristensen 2000) that
used burrowing filter feeding Polychaete worms and found that the
oxidation of older, deeper organic matter along the burrow's walls had
an increased CO² production of 47% and a mineralization of the old and
partly degraded organic matter around the burrows was enhanced by up to
a factor of 10 when exposed to oxygen. All to the benefit of the
macroalgae.
Coral Rubble Habitat 
With the amount of coral
rubble
present and its weathered appearance, it is apparent that
decades
ago this area held substantial shallow water coral reefs. I can only
speculate that the shift from coral dominance to algae dominance was
the result of human development of the island resulting in increased
amounts of nutrients being washed into the ocean during the monsoons
and its heavy rainfall.
Buried within the sandy sediment are numerous coral and limestone fragments. The
surface rubble is quite extensive and forms a layer averaging 8cm
thick providing the macroalgae and infauna a relatively stable substrate on
which to attach and live amongst..
With its relatively large gaps, the coral rubble provides numerous
small tunnels and voids in which organic detritus accumulates while
also creating sheltered habitat for numerous infaunal species, the most
populous being worms and amphipods. Such high numbers of
available prey makes this area a favorite hunting ground for transitory
reef fish, most notably the members of the Wrasse family that move into
the area during periods of high tide and are capable of flipping
over and through the coral rubble. These daily disturbances can
prevent the growth of algae onto the substrates.
The Rock Habitat

As a stable structure, the calcium carbonate rocks
that are found in abundance within the subtidal zone create a
sheltered habitat for many animals while providing a solid substrate
for the macroalgae to grow upon, providing yet another habitat that in
combination with the rock makes for a very diverse community of both
algae and animal species.
Calcium carbonate based rock is very porous simply by the nature in
which it was created. A number of invertebrates, collectively
called cryptofauna, inhabit the coral rock substrate itself, either
boring into the limestone surface or living in pre-existing voids and
crevices. Those animals boring into the rock include sponges, bivalve
mollusks, crustaceans and Sipunculans, there are of
course numerous other life forms that are
capable of burrowing through the rock or enlarging smaller tunnels that
run through the rock and in doing so, the many cryptofauna keep
such tubes and tunnels clear of algae and sponge growth that would
quickly block off any interior structures and prevent the bacterial
nitrification and denitrification within the porewater that is exchanged very slowly by the
cryptofaunal movement through the tunnels and pores of the rock
while providing nutrients to the porewater through
respiration and the creation of waste products through their own
feeding, thus providing a localized source of nitrogen and
phosphorous to
the epiphytic macroalgae.
The most commonly found and most
visible of the cryptofauna are the marine worms, the majority of which
are scavengers and predators, consuming detritus matter yet quite
capable of becoming predatory when the chance arises.

The Eunicids are the largest and most numerous of the predatory
worm species comprising 60% of the worm species that I find living
within the rock's tunnels, of which they never fully exit from yet extend
outwards from their tunnel onto the sediment or up into the
macroalgae in search of food. They are also capable of extending
their range or reach by creating tunnels from small rock fragments that
they "glue" together in order to move farther afield while remaining
under cover and protected from predators. In doing so, the
Eunicids are credited with creating or extending coral reefs as the
corals find such structures suitable to settle upon and create new
coral colonys.

The
Amphinoids are another common predator and scavenger within and amongst
the rock structures appearing to be much more mobile traveling between
rocks in search of their food. This apparent freedom to roam
openly is most likely due to their being very well defended by
their calcareous
setae that are filled with poisonous secretions, hence their commonly
being called the fire worms.

The Cirratulids are deposit feeders which gather food by
means of their palps. They are sluggish worms with varied life
styles, some species bury themselves
below the surface of sea bottoms leaving only their gills and palps
visible. Some are free-living and inhabit tubes, while others are
capable of burrowing through corals, shell or rock. They occur
both in shallow and deep sea areas.

The Thalassinidean shrimp account for the creation of many of
the larger burrows that run through the rock remaining very cryptic
only venturing to the openings of their burrows to feed upon detritus.
Their constant movement of the water and their respiration
account for a large portion of the nutrient processing as their
oxygenated burrows provide for aerobic bacteria.
There
are of course a great many other species that use the rocks as shelter,
feeding and hunting grounds creating a micro-habitat unto themselves
that when multiplied by the countless rocks found in the subtidal
zone creates a substantial and very diverse nutrient web.
For more photographs of the life forms that I have been able to
document
living within and on the rocks found in the subtidal zone, please
see these pages.
Macroalgae Epifauna
The
macroalgae themselves support a number of fauna species by just their
structures creating a habitat in which epifauna can eat and live
within. Algae morphology plays a large part in the population
densities of epifauna. Tall, stick like algae provide little
cover for mobile epifauna making them easy prey while the thick, bushy
or mat forming macroalgae species provide safe haven from large
predators such as fish. Very few species are found as epiphyts as
the macroalgae do not provide a long term stable substrate on which
to grow upon.

With the vast amount of vegetative matter to be found in the
subtidal zone, there are vast amounts of herbivore species taking
advantage of such bounty while others find the vegetative cover and its
taller structures a suitable substrate on which to settle and grow,
however temporary such substrate may be. Of course where there
are herbivores, there are the predators.
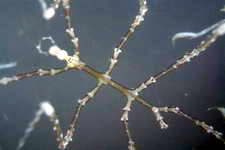
The Sea Spiders are a large group containing many species, some
much larger than others but all are predatory, stalking their prey
amongst the macroalgae.

Snails make up the largest percentage of herbivores found
amongst the macroalgae covered rock substrates with a very large
diversity of species, from the thumb nail sized cowrys to the four
inch long abalones. Predatory Whelk species are also very common
as they seek out and feed upon the herbivore species.
The Benthic Macroalgae
- Primary Producers
Benthic macroalgae are those algae that project more than 1cm
above the substratum and are photosynthesizing multi-cellular
organisms
that lack the specialized structures and reproductive mechanisms
characteristic of true plants, lacking the true leaves, stems,
and
roots of plants but have similar parts that include the thallus,
blades, pneumatocysts, stipes, and holdfasts. The thallus is
the "body" of the macroalgae and includes the blades, stipe,
and
pneumatocysts. The blades are the leaf-like portions of the thallus and
the stipe is the stemp-like structure that provides support.
The
pneumatocysts are gas-filled bladders that are present on some
macroalgae to help keep the blades near the surface to maximize
photosynthesis. The holdfasts are structures that look like roots yet
function only as a means to attach the thallus to the substrate, in most species that is.
 Chlorophyta
Chlorophyta -
Unlike the red and brown macroalgae, most green macroalgae are found in
freshwater or terrestrial environments. Of the roughly 8,000 species,
only 10% are marine. However, the species of green
macroalgae that are found in the marine environment can be very
abundant by sheer biomass.
Most of the green algae species are structurally
simpler than the red and brown macroalgae, many of which are
unicellular Some are even planktonic and use flagella to
swim! Green algae derive their color from the pigment
chlorophyll, used during photosynthesis.
Many common green macroalgae are
multinucleated, some where
in between unicellular and multi-cellular. Green macroalgae may not have
the typical macroalgae structures such as a thallus
or holdfasts and
instead may be segmented, spherical clusters, branching and
tube forms
or calcareous branches.
 Rhodophyta
Rhodophyta -
With roughly 800 genera and 5,200 species there are
more red
macroalgae species in the oceans than there are of green and brown
macroalgae
combined. Most species are red or pink in color, the result of red
pigments called phycobilins that mask the green color of the
chlorophyll in their cells. Most red macroalgae are marine and live in
shallow-water environments.
Their structures often
vary by the energy of their
environment, in strong wave action the algae are often low and
encrusting, whereas in deeper waters the algae are more
substantial with long branching blades. Coralline red algae
are
important contributors to the formation of reefs and to sandy
sediments. They do this by secreting calcium carbonate within
their
cell walls for protection and support.
 Phaeophyta
Phaeophyta -
The brown macroalgae usually varies from olive green to dark
brown in
coloration. Their coloration is derived from an abundance of
yellow-brown fucoxanthin pigments which masks the green color of the
chlorophyll in their cells.
Most brown macroalgae are marine with roughly 2000
species.
Morphology - Simple yet Functional
The
macroalgae are a morphologically diverse group having great variability
in form and function. With numerous studies having been done on
their ecological and physiological performance it has become evident
that the functional form of the thallus accounts for much of the
variability. The surface areas of the thallus are not only
responsible for the gathering of light used in photosynthesis but are
also used for nutrient uptake. The
paper-thin
Ulva spp. have a
thallus that is only 1 or 2 cells thick, exposing all cells to light
and nutrients while other macroalgae genera can be a few centimeters thick
and the majority of their cells are not in contact with the surface.
These differences in thallus shape, size and thickness determines
not only the effectiveness of macroalgae at gathering both light and nutrients but
play a large role in their being able to survive long term limitations.
Those algae with a very thin thallus tend to be restricted to
nutrient enriched sites as they have little nutrient storage
capacity whereas those algae with a thicker thallus allow for the development of
strategies to endure in low nutrient sites.
Morphological forms while trying to serve nutrient and
photosynthetic functions are also determined by numerous other
factors. A good species example are the
Halimeda, commonly
found on the coral reefs and subject to numerous herbivorous fish
species, such threat has been met with calcified growth in order to
deter most herbivores allowing the algae to endure where few other
algae can. Something as seemingly simple as wave action and water
velocities can also determine local species composition with only those
species that have developed the means to either anchor themselves with
stout holdfasts or have an encrusting growth form are able to survive
the physical stresses of surfs and tides. Include competition
from other algae, chemical defenses, variations in irradiance,
temperature and salinity and one can begin to understand why, that even
within the same genera there are vast differences amongst the species.
Growth & Reproduction
- Ramets, genets and modules oh my!
With
simple modular morphology yet complex life cycles, the
macroalgae represent one of the great success stories of life on our
planet, having colonized the oceans, lakes, streams and the land.
Their ancient modular form may appear simplistic yet in
reality
they lead a complex life style capable of multiple methods of
reproduction and producing many complex compounds, many of which are
found in the terrestrial plants that have evolved from marine algae.
The morphology of the macroalgae is based upon a modular cell
arrangement. These cellular units are functional,
semi-autonomous, ecologically interactive and reproductive
(vides
2002).
Clonal growth
in algae is present within the three main algal divisions. Stoloniferous
types
(Fig. 2, A&B)
of growth manifests itself in the thallus (i.e. plant body)
consisting of a prostrate creeping system (stolon) usually
exhibiting
apical growth and giving rise to an erect system, which is
usually branched. Asexual
propagation can occur
(Fig.2 C)
by means of algal fragments that re-attach to the substrate
or by the bending of erect branches
(Fig.2 D)
and the re-attachment of the cell to the substrate,
initiating the growth of a new thallus. Common holdfast growth
(Fig.2 E) is achieved by the production of
an initial, prostrate, basal system from which one or more erect thalli
may develop. Crusts are
also a clonal mode of algal growth, characterized by apical
activity at the margin of the plant body resulting in a horizontal
cover of the substrate without any erect axis.
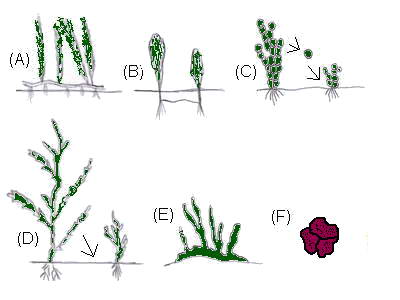 Figure 2.
Different types of clonal growth. (A) Stoloniferous
growth.
(B) Buried stolon. (C) Fragmentation (ramets).
Figure 2.
Different types of clonal growth. (A) Stoloniferous
growth.
(B) Buried stolon. (C) Fragmentation (ramets).
(D)
Branch bending and re-attachment. (E) Fronds growing from a
holdfast. (F) Crustouse growth. The
Modules
The
Modules - Building blocks of the algae.
All clonal macroalgae are sessile, branched organisms with a
hierarchical organization. A module is simply a repetitious
multi-cellular unit and can be defined "
as a result of the apical (the
tip)
, basal or
intercalary cell that is constantly dividing"
(Vides 2002).
The bulk mass of the algae is the result of a change in the
plane
of division of the module which can result in upright
structures
or horizontal encrustment.
The module
is either a filament branch
(Fig 3. A), or
a coenocytic branch, multiple nuclear divisions without accompanying
cell divisions
(Fig3. B),
or an organized collection of loose cells contained within a membrane.
(Fig 3. C).
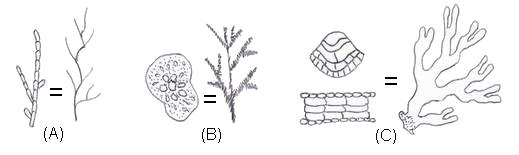 Figure 3.
Different levels of module organization in marine macroalgae.
(A) Uniseriate branched filament
Figure 3.
Different levels of module organization in marine macroalgae.
(A) Uniseriate branched filament
(B) Medullar or
pseudo-parenchymatouse organization. (C) Parenchymatouse
organization.
- Fragmentation Reproduction.
Some modular
algae have the ability to disintegrate into
ramets and
give rise to plant parts that behave as independent organisms
.
This ability to
fragment into ramets is a very successful survival and reproductive
method as any disturbance (i.e. herbivores, storms) that fragments or damages
the algae can aide in its dispersal. A trait that is very
common
in all algal divisions. Small fragments of the calcified, branched
green algae
Halimeda
(Walters et al. 1994) and the red algae
Corallina (Littler
et al.1984) have
the ability to reattach to the substrate and form a new individual
which functions as an independent unit. This is also
true for
the broken branches in many fleshy red algae
. Since
algae lack any "organ" differentiation, any module branch has the
potential to become an independent individual.
Life
Cycles - Sexual Reproduction Methods.

Its all about meiosis, a two-stage type of cell division in sexually reproducing organisms that results
in the development of sperm and egg cells. In meiosis, a
diploid cell divides
to produce four
haploid cells , each with half the original chromosome content.
In organisms with a diploid life cycle, the products of meiosis are called
gametes. In organisms with an alternation of generations, the products of
meiosis are called spores.
 Chlorophyta Reproduction
Chlorophyta Reproduction
Illustration © Worth Publishing, reprinted with permission
The Chlorophyta can reproduce Asexually by fission (splitting), fragmentation or by
zoospores (motile spores). Sexual reproduction is very common and may
be Isogamous (gametes both motile and of the same size), Anisogamous (both motile and
of different sizes) or Oogamous (female non-motile and egg-like,
male motile). May have an alternation of haploid and diploid phases. The haploid
phases form gametangia and the diploid phases form
zoospores by meiosis. Some species do not have an alternation of
generations, meiosis occurring in the zygote.
 Phaeophyta Reproduction
Phaeophyta Reproduction
Illustration © Worth Publishing, reprinted with permission
The
Phaeophyta have an alternation of haploid and diploid
generations. The haploid thalli form isogamous, anisogamous or oogamous gametes
and the diploid thalli form zoospores by meiosis. The haploid and diploid thalli may be similar (isomorphic) or
different (heteromorphic) in appearance.
 Rhodophyta Reproduction
Rhodophyta ReproductionIllustration © Worth Publishing, reprinted with permission
The
Rhodophyta usually reproduce sexually. Sexual reproduction is oogamous
involving non-motile spermatia and closed mitosis. Tetraspores are produced in
the tetrasporangia during meiosis. If asexual reproduction occurs, it does so
through
aplanospores.
Lighting Requirements - Usage and Adaptation
Light is the energy source for the conversion of inorganic carbon to
organic matter by photosynthesis, making light the
prerequisite for the vast majority of all life on our planet as
most food chains or webs begin with vegetative matter.
Tropical macroalgae occupy regions of the world that receive the
highest levels of photosynthetically active radiation (PAR) and
ultraviolet (UV) irradiance, reaching levels five times higher than that which macroalgae require to obtain photosynthesis
saturation and can cause photoinhibition
(Beach 1997). With
few, if any locations providing the "perfect" environment, the
macroalgae just as any other organism in the ocean, must be able
to adapt to variable conditions or face being restricted to greatly
reduced ranges or extinction. With their greatest diversity found in the tropics
the macroalgae have had to develop not only the means to protect
themselves from over-exposure but also the ability to adjust the
amounts of their defensive pigmentations, such defenses are variable
among the three algal divisions. It is by these adaptive
and protective capabilities that the macroalgae have spread far and
wide.
All
photosynthetic algae and plants use pigments to capture the energy
of
light. The captured energy must be transferred to a molecule of
chlorophyll "A" before it can be used, making chlorophyll "A"
the standard amongst the algae.
The different divisions of macroalgae have additional chlorophylls
along with other pigments used to gather the light that they need
or to block any excess
before damage is done.
Rhodophyta
Phaeophyta ChlorophytaPhotosynthetic pigments -
Chlorophyll A & D
Chlorophyll A & C
Chlorophyll A & B
Rhodophya contain the pigment
phycoerythrin
which gives them their red colorations. Phycoerythrin reflects red
light and absorbs blue light. With this ability to utilize the blue
wave length better than the other algal divisions, they are able to
photosynthesize at slightly greater depths since blue
light penetrates water
farther than light of longer wavelengths, this ability to utilize the
blue wavelengths of light also enhances their photosynthetic capability
when growing in shallow water and are being shaded by their own or
their neighbors growth allowing even the lowest sections of the algae
to contribute.
Phaeophyta have large amounts of
carotenoids,
and it is these brown and golden pigments which give the brown algae
their characteristic color. The most important carotenoid in the
phaeophytes is
fucoxanthin.
Having additional pigments that utilize the yellow/green wavelengths of
light provides nearly the same capability to photosynthesize while
being partially shaded as the red macroalgae have developed.
Chlorophyta
having the most common of the chlorophylls and very few protective
pigments that allow
long term exposure to intense sunlight or the ability to use other
light wavelengths, the green macroalgae have to make up for what seems a
shortcoming by simply growing faster than any damage being done while
often restricted to shallow, near shore areas receiving intense
levels of sunlight, appearing to trade off any possible light induced
risks in favor of the near shore areas that often equate into higher nutrient
levels.

The accessory pigments capture wavelengths of light that chlorophylls cannot
and transfer the energy to the chlorophyll which uses this energy to carry out
the light reactions. These pigments are arranged in the thylakoid membranes in
clusters, along with proteins and electron carriers to form
light-harvesting complexes referred to as photosystems. Each photosystem has
about two hundred chlorophyll molecules and a variable number of accessory
pigments, the type and amounts vary within the algal divisions.
At
the center of every chlorophyll molecule is
a reaction center
to which all the other pigment molecules pass the energy they harvest
from sunlight. When the reaction center's chlorophyll absorbs light or
receives light energy from its accessory pigments, its electrons
become excited, these electrons carry the energy from light
and pass it to an electron acceptor molecule making usable energy
available to process the many functions needed to sustain the
macroalgae, most notably, carbon fixation.
While light is of course, very critical to the photosynthetic process, the dark period is also very important
(Young 1997).
It is during this dark period that some of the excess light intensity
received during daylight is put to use to drive other processes.
It is within this second stage of photosynthesis that the energy
released from
ATP drives the production of organic molecules
from carbon dioxide.
As
a type of stored energy, this process has a limited duration and
will cease to function in prolonged periods of darkness. While it may
not appear important that photosynthetic algae would require a period
of darkness, a recent study of microalgae (
Rost 2006)
revealed that rates of photosynthesis and carbon uptake increased
dramatically in a cycle of light/dark compared to continuous light in
two of the three species examined.
In addition to having a biological need for periods of darkness, some
macroalgae species have been found to use the cover of darkness to do
most of their growing
(Hay 1988), producing new, more
herbivore susceptible growth
at night when herbivorous reef fish are
inactive. New growth has 3 to 4 times the food value than older portions of the algae. The
Halimeda spp.
are good examples of using this strategy to deter herbivores, in
addition to trying to sneak in new growth during darkness, the algae
concentrates its chemical defenses into new growth making the most
nutritionally appealing parts the most noxious. As the new growth ages,
their morphological defense increases through calcification while
becoming less nutritional. Additionally, new growth of
Halimeda
remain unpigmented until just before sunrise thus saving the nitrogen
containing molecules used in photosynthesis for when light is available
and they can start producing food and energy.
Growing in shallow waters where environmental changes often occur
quickly, the reef flat's macroalgae are exposed to stress factors such
as changes in irradiance, salinity, temperature and desiccation,
requiring quick responses to survive sudden extremes. Changing
irradiance levels require responses to both subsaturation (not enough) and
supersaturation (too much) of light.
At lower irradiance levels the photosynthetic rate is limited by
the irradiance, but at higher irradiances above the algal saturation
points they are absorbing excessive light and energy. Excess
energy can lead to photoinhibition reducing photosynthesis through the
formation of reactive molecules such as singlet oxygen, superoxides and
hydrogen peroxide. These molecules not only reduce efficiency but
can cause extensive damage to the photosynthetic complexes and to the
chloroplast membranes of which the algae have had to develop mechanisms
to prevent. Among the most important protective mechanisms
against excess light is the xanthophyll cycle.
The xanthophylls are yellow accessory pigments based on the carotenes. The
Rhodophya and
Phaeophyta divisions
carry these accessory pigments which are involved in photosynthesis
along with green chlorophyll. The xanthophyll cycle converts
pigments from a non-energy inhibiting form to energy inhibiting forms
to reduce the absorption of light energy received and thus protecting
the chlorophyll's reaction center.
The
mechanisms and processes involved with photosynthesis are extremely
complex with the differing algal divisions having solved a host of
problems associated with life under our sun and have done so in similar
yet slightly different manners that allows each division a niche to
fill.
Carbon - Utilization and the role of pH
In seawater with a salinity of 35 psu, the concentration of
dissolved inorganic carbon (Ci) is about 2.1 mM. At a pH of about
8.0, the majority of dissolved inorganic carbon is in the form of
bicarbonate (HCO³) with only 12 µM of carbon dioxide
(CO²). Macroalgae are able to utilize both CO² and HCO³
for photosynthesis. Since carbon dioxide is an uncharged molecule
it readily diffuses into algal cells making it instantly available to
the macroalgae for fixation, but with relatively low amounts of
CO² available in seawater, environmental factors such as
low water flow around dense mats of algae can easily make CO²
limiting for photosynthesis. While CO² uptake requires
little, if any effort by the macroalgae it is not a
reliable source of carbon for the macroalgae.
H²O + CO² <---> H²CO³ <---> H+ + HCO³ <---> 2H+ + CO²¯³
Carbon dioxide in sea water is in equilibrium with air levels of carbon dioxide and results in various forms of carbon.
The proportional levels of the various carbon forms are pH dependent. At low pH, a larger proportion is present in the
form
of carbon dioxide and the reactions to the left are favored.
At low pH, carbon dioxide is so abundant that it
drives
photosynthetic rates by direct diffusion (at no energy cost)
into the algal cell. At much higher pH values the
majority of carbon is present as bicarbonate and carbonate with little carbon dioxide available.
Since natural sea water's pH creates a high prevalence of bicarbonate, all three
algal divisions have developed the means to
uptake the more readily available bicarbonate, some species better than others
(Klenell 2004)
and do
so by creating an acidic zone in the periplasmic space between the
chloroplast and the cell's wall that reduces the bicarbonate into
carbon dioxide. Through the use of alkaloid inhibitors, researchers
have been able to determine that the macroalgae have
developed internal proton pumps capable of manipulating pH levels to
their advantage. The processes involved are very complex and beyond the
scope of a single article.
As shown above, pH determines the availability of carbon dioxide
and one might get the impression that a lower pH value in sea water
would be beneficial to the macroalgae as more readily available carbon
dioxide would be present. This however is not the case as shown
in growth and carbon uptake studies done to date. In one of the studies
(Israel 1999), both optimal growth and maximum photosynthesis of
Porphyra linearis
occurred at about the pH and inorganic carbon levels of natural
seawater. At pH extremes (e.g. 6.0 or 9.0) photosynthesis was
inhibited which of course resulted in slower growth. Low pH increased
the dark respiration rates and consumed the previous light
period's photosynthate leading to less growth. At higher pH levels
photosynthesis did not simply slow down, but ceased altogether,
suggesting that the oxygen evolution of the thalli were greatly
affected.
Also of interest are the studies that have been done showing that those
algal species that are not very adept at utilizing HCO³ may enhance their limited ability by taking
advantage of close relationships with CO² producing
organisms, allowing epiphytic organisms to grow upon themselves in
order to utilize the CO² that all animals create through
respiration, such as bryzoans. Such epiphytic growths would
normally be considered a detriment due to shading but that concern has
been shown to be partially invalid as sufficient light intensity can pass through
some species of bryzoans to stimulate photosynthesis. A second
means of supplying CO² to macroalgae is the sedimentation of
particulate organic matter onto the macroalgae with subsequent
microbial activity generating CO². At one time such growths
and deposits onto macroalgae were perceived to be entirely negative
due to shading of the macroalgae
(Raven 2003).
Macroalgae & Nutrients
- Nitrogen vs phosphorus-limited growth.
In shallow coastal waters, the availability of nutrients
controls algae performance and species composition. These algal
communities contain a large number of species that represent
various growth strategies and life cycles. The two most commonly
found growth strategies on the reef flats are the attached (Haptophytes) slow growing species and the unattached
ephemeral species.
Near complete coverage of the sediment by thick mats of
macroalgae shifts
the nitrogen processes normally associated with sediments upwards into
the lowest areas of the algal mat above the
sediment, suppressing the sediment's ability to perform nitrification
and denitrification. The algal mat's creation of a lowered oxygen zone
below the sunlit canopy performs the same amount of
denitrification as bare sediments
(Krause-Jenson 1999).
Nitrification is also suppressed near the sediment as the
NO³ from the water column is efficiently assimilated by the
algae
and nitrification is absent due to the anoxic conditions near the
sediment. It is the creation of this microenvironment that allows
the slow growing, mat forming macroalgae to persist within
oligotrophic water columns.
With the onset of the wet season and its
storm
induced disturbances, the algal mats can be torn away from the
substrates bringing about rapid change and returning the
processes of
nitrification and denitrification to the sediments only to shift back
again as the algae regain sediment coverage. As such, the
role of the sediment is still an important factor. With the
sediment providing localized nutrients, the macroalgae that settles
within the area has a ready source of nutrition until it forms large
enough mats to once again take over as a primary consumer and producer.
In
eutrophic reef flats with large areas of macroalgal cover, the physical
structure and growth stage of the algal mats may play a large role
in nitrogen removal by assimilation and
denitrification, cleansing the water of nutrients before it can
reach the coral reef.
Typically the
macroalgae are distributed along nutrient gradients with the slow
growing, mat forming species normally found in nutrient poor regions and the fast
growing ephemeral
species
becoming dominate under nutrient-rich conditions. These
observations lead me to believe that the larger, slow growing species
have a slower rate of nutrient uptake than the smaller, faster growing
species that have a higher nutrient demand and higher rate of
growth, or so I thought.
When two slow growing and four fast growing species were examined
(Pederson 1997)
for their ability to sustain growth during low nitrogen levels it was
found that the nitrogen required to support maximum growth varied
16-fold amongst the species, with the fastest growing algae having the
highest demand, as was expected. The fast growing species
took up nitrogen 4 to 6 times faster per unit of biomass than the
slower growing species at both low and high substrate concentrations,
but the ratios of maximum nitrogen uptake to requirements were larger
among the slow growing algae.
Previous studies have shown that the fast growing species were able to
assimilate nitrogen faster due to their higher rate of growth. But
while the larger and slower growing algae may be better suited to meet
their nitrogen requirements at low levels, Pederson's study reveals
that sudden surges of nitrogen are met with the same growth and
nutrient uptake response by both the slow growing and fast growing
species, suggesting that surge uptake is of a minor ecological
importance. What becomes important per species composition is not
the level of nitrogen eutrophication, as all species respond in kind,
but the duration of it that determines the species composition of an area.
From surveys
(Larned 1998)
of nutrient-enrichment studies using tropical macroalgae it became
apparent that it is not possible to use broad generalizations about
growth-limiting nutrients as 39 species tested within seven studies
showed inorganic nitrogen enrichment enhanced growth in 22 species and
inorganic phosphorous enhanced the growth of 17 species. There are no
distinct patterns of nutrient limitation within the three algal
divisions
making nutrient limitation a species specific occurance. It is however
worthy to note that the majority of macroalgae species tested showed a
preference for NH4 when in higher concentrations than NO3, which makes
physiological sense as NO3 uptake is partially dependent upon
photosynthesis and costs energy.
| Species | Assay | N | P | N+P | DIN:PO4 |
| Ulva fasciata
| G | + | 0 | 0 | 4.9 |
| Dictyosphaeria versluysii | G | + | 0 | 0 | 4.9 |
| Codium edule | G | 0 | + | 0 | 4.1 |
| Caulerpa sertularioides | G | + | 0 | 0 | 4.5 |
| Caulerpa racemosa | G | + | 0 | 0 | 4.5 |
| Dictyosphaeria cavernosa | G | + | 0 | 0 | 4.2 |
| Halimeda opuntia | P | + | 0 | na | 18.5 |
| Sargassum echinocarpum | G | + | 0 | 0 | 4.1 |
| Padina japonica
| G | + | 0 | 0 | 4.1 |
| Dictyota divaricata | P | 0 | + | na | 18.5 |
| Hydroclathrus clathratus | P | + | + | na | 3.1 |
| Kappaphycus alvarezii | G | + | 0 | 0 | 4.2 |
| Gracilaria salicornia
| G | + | 0 | 0 | 4.9 |
| Acanthophora spicifera | P | 0 | + | na | 18.5 |
| Gracilaria tikvahiae | G | + | + | + | <10 |
| Laurencia poitei
| G | 0 | + | 0 | <10 |
Assay: G tissue growth, P photosynthesis (oxygen evolution)
For N and P
treatments: + main effect of nutrient enrichment significantly
greater than control
- significant inhibitory effect of nutrient
enrichment 0 effect of nutrient enrichment not significant
For
N+P treatments: + significant positive interaction - significant
negative interaction 0 interaction not significant na not applicable.
Source - Larned 1998
MicroNutrients
As the name implies, they are those elements that occur in
extremely small (trace) amounts. Over sixty elements have been found in
the tissues of macroalgae, some are of great importance to the algae,
others are sequestered toxins with no known use. In natural
environments it is highly unlikely that micronutrients would ever
become limiting to the macroalgae simply because of the minute amounts
required and there being sufficient bioavailable forms in
natural sea water, with the exception of Iron that is most
prevalent in its insoluble form (Fe3).
Iodides are found concentrated in the tissues of marine macroalgae in
both their inorganic and organic forms. However, the mechanism(s)
by which macroalgae concentrate iodine from seawater or store it are
not understood, even the role of iodide in seaweed is unknown yet
most macroalgae contain large amounts of both organic and inorganic
iodide compounds. Studies have indicated that the chemical species and
contents of iodine in various algae are remarkably different. The
highest iodine content of 734 mg/kg (wet basis) was found in
Laminaria japonica, with 99.2% of the total iodine being water soluble.
The iodine contents of other macroalgae are lower and soluble iodine makes up
16-41% of their totals, suggesting that
the mechanism of iodine enrichment is different for various algae and that its
bioavailability varies as well
(Hou 1977).
Iron (Fe) is an important nutritional element for
phototrophs because
of its requirement during chlorophyll synthesis and is an important
regulatory factor involving many biochemistry aspects pertaining to
the enzymes of nitrogen assimilation, photosynthetic carbon
fixation and cell maintenance
(Liu 2000). Prolonged
iron deficiency not only reduces the efficiency of the algal energy and
nutrient pathways but also begins to affect the very structure of its
chloroplasts and quickly reduces pigment content inhibiting the
transfer of light energy to the chlorophylls. The reduced
pigments and inefficient chlorophylls also make the macroalgae
vulnerable to light induced damage resulting in
chlorosis and eventually the death of the algae.
Manganese (Mn) is the
second most important element required for photosynthesis and exists in
the aquatic environment in two main forms: Mn(II) and Mn(IV).
Movement between these two forms occurs via oxidation and reduction
reactions that may be abiotic or microbially mediated. The
environmental chemistry of manganese is largely governed by pH and
redox conditions; Mn(II) dominates at lower pH and redox potential,
with an increasing proportion of colloidal manganese oxyhydroxides
above pH 5.5 in non-dystrophic waters
(Raven 1999).
Just
as some steps in the chlorophyll synthesis pathway are iron dependent,
similar pathways depend upon manganese to convert protoporphyrin
to protochlorophyllide, both of which are light capturing molecules (chromophores) and essential for efficient photosynthesis.
The macroalgae have use of many
of the trace elements found in sea water yet are able to sequester much
greater amounts than needed for biochemistry functions. This ability to
store above normal levels has not gone unnoticed by science and many
common species of macroalgae have been used for more than 30 years
to indicate and monitor the metal pollution levels in marine
environments. That same ability also makes it difficult to determine
what are, or what should be natural trace amounts in the ocean as
man-made terrestrial sources add great amounts of metals to the oceans
each year, so what was or is now, naturally occurring levels?
In a study to determine trace metal levels within a number of macroalgae species
(Villares 2005), samples
of algae were collected from estuaries located both near and
far from human population centers and not only showed
evidence that all three macroalgae divisions sequester metals but that
different species have different storage capacities for the various
metals, below are a few examples of the study results.
Species
& Location
Cobalt Chromium Copper Nickel
Lead Zinc
Ascophyllum nodosum Reference
5.40 13.79
37.52 14.44
3.57 19.92
Contaminated
5.79 26.82 179.50
43.91 3.71 48.15
Enteromorpha spp.
Reference
8.12 19.41
104.53 25.33
9.32 57.65
Contaminated
11.42
29.27 212.65 42.63
16.59 67.02
Fucus ceranoides Reference
5.85 5.39
57.74 9.09
1.12 30.13
Contaminated
8.52
4.48 36.31
9.48 3.11
69.30
The excess of any metals, including those of use to the macroalgae can
become toxic and interfere with all life functions. Given
the above data the macroalgae obviously have methods to deal with some
excess, which range from transporting the metals elsewhere in the
plant, compartmenting the metals or by creating compounds
that reduce the metals to their less toxic forms. All of this
would obviously cost the macroalgae both energy and resources better
spent on growth and reproduction but does allow the macroalgae to
survive. The limits of tolerance or the ability to deal with
excessive amounts are species specific
(Bertrand 2005) Temperature & Salinity
The tropical macroalgae have adapted to life on the reef flats and
the environmental variations brought about by the change of the seasons.
With the calm and sunny dry season, consistently high levels of
irradiance provides ample energy for photosynthesis yet those same
irradiance levels also create consistently higher water temperatures.
Six months later and with the arrival of the monsoonal rains,
irradiance levels are greatly reduced due to cloud cover resulting in
consistently lower water temperatures in comparison to the dry season.
Of the primary physical factors that influence macroalgae growth, water
temperature is the most important factor in the geographic
distribution of tropical macroalgae while salinity is one of the most
critical chemical factors affecting their local distribution.
Both temperature and salinity influence photosynthesis and
respiration.
The macroalgae species that are found nearest the shoreline (intertidal) and
its shallower water depths experience the greatest fluctuations in
water temperature and salinity between the two seasons while those species that are
found at greater depths (subtidal) due to their ability to better utilize reduced
irradiance levels experience less dramatic temperature and salinity variations.
Below is but one example of how the tropical macroalgae species
are able to adapt to changing conditions. While each species may have
different optimal conditions, there are numerous studies showing that
in general, the tropical macroalgae as a whole, can tolerate the
temperature and salinity changes that are brought about by the changing
of the seasons.
Gelidiella acerosa is
a common component of macroalgae communities in Philippine coastal
waters and makes a good case study on the effects of varying
temperature and salinity levels as it is found from the upper
intertidal to the shallow subtidal areas, as well as in tidepools near
the seaward margin of fringing reefs
(Kain 1999). In Kain's study, samples of
Gelidiella acerosa
from intertidal, subtidal and tidepools were subjected to four salinity
(22, 28, 34, 40‰) and three temperature (22, 28, 34°C) combinations. The upper intertidal plants tolerated low
salinities (22–28‰) better than high salinities
(34–40‰), while tidepool and subtidal plants were not
affected. Temperatures of 22 through 34°C resulted in a one-fold
increase in their photosynthetic rates and insignificant differences in
their respiratory rates while tidepool and subtidal plants almost
doubled their photosynthetic rates and their respiration rates
increased by about 5–50 times. Intertidal plants appeared to be
more tolerant to wide temperature fluctuations and low salinity levels,
while tidepool and subtidal plants were least affected by salinity
variations but were quite sensitive to temperature fluctuations.
Vegetative and
tetrasporic
plants had similar photosynthetic and respiratory responses to salinity
and temperature variations, although vegetative plants had
significantly higher net photosynthesis under the minimum and maximum
temperatures tested (22 and 34°C). Reproductive
G. acerosa
showed greater tolerance to temperature fluctuations. These responses
indicated that physiological changes may have occurred when the species
became reproductive
(Kain 1999).
In
other temperature tolerance studies it has been shown that
fluctuations in temperature creates a positive growth rate in
macroalgae.
Ulva pertusa
at an average temperature of 20°C showed significant growth rates
with temperature fluctuations of 2 - 6°C compared against a
constant temperature. Chlorophyll-a and protein at +2°C and +4°C were slightly higher
than those at the constant temperature of 20°C, which indicates
that
the synthesization of some biochemical products were accelerated at
temperatures slightly higher than average. However, a fluctuation of
8-10°C from the average did not show any positive influence on
growth, showing that diel temperature fluctuations do exert
various influences on the biochemical composition of the algae to some
degree
(Wang 2007).
Chemical Defenses - An arms race.
As defensive measures against herbivores, the macroalgae have
developed a great number of defensive chemicals and responses against being
grazed upon. Such defenses influence herbivore preferences and can
indirectly influence algal community structure. Should select
species of herbivorous fish be reduced in number, the remaining
herbivores would continue to graze their preferred algae while leaving the
unpalatable algae free to dominate having had their grazers removed or reduced.
Most studies done to
date on chemical deterrents have focused on fish and sea urchin
herbivores as they are the most prevalent herbivores found on coral
reefs and reef flats. I am unaware of the effects that algae
produced chemicals have upon other herbivores such as the gastropods,
but I suspect that since most gastropod herbivores have preferences in
their grazing as well, the chemical deterrents
most likely play a role in their dietary choices also.
The array
of defensive chemicals is quite impressive,
including polyphenolics, acetogenins, terpenes, amino-acid-based
and halogenated compounds
to name just a few, all of which not only influence palatability but
are also produced to protect against microorganism infections
(Engel 2006), to ward
off space competitors (allelopathy) and to keep themselves clear of
epiphytic growth.
The
Rhodophyta,
in recent years has generated interest in their unique ability to
produce hundreds of different halogenated organic compounds, each
chemical species being produced by but a few algal species within the
division. Just as the sponges and other organisms have gained attention
from their chemical products being of possible use in the medical
field, so has this algal division. Many new studies are being generated
due the recent discovery that acyclic monoterpene
halomon produced by the red macroalgae have selective anti-tumor
properties in humans
(Kladi 2004).
The
Chlorophyta have been found to be even less palatable than the other divisions. Most notable are those algae of the order
Caulerpa, Halimeda and
Bryopsidales (Meyer 1994).
This may be attributable to algal activated defense systems, a
means in which the algae are able to rapidly convert stored secondary
metabolites into chemicals with greater bioactivity when the algae is
wounded as occurs when grazed upon. This rapid response to grazing is
an effective means in which to deter a herbivore that does not find the
normally present compounds to be of deterrence. Examples are caulerpenyne from the
Caulerpa spp. and halimedatetraacetate from
Halimeda spp.
which upon wounding are transformed into the more toxic oxytoxins
and halimedatrial. These are but a few more examples of the
numerous compounds that the algal divisions produce to deter herbivores.
The
Phaeophyta
has the distinction of being the only algal division that produces
phlorotannins, which are polymers of phloroglucinol, a class of
polyphenolic compounds that make up 1–20% of the algal dry
weight
(Luder 2004). They occur as secondary
metabolites in a wide range of molecular sizes and have an
astringent taste, bind to metal ions and precipitate proteins.
Luder's microscopic study showed results suggesting multi-roles for
phlorotannins during wound-healing, wound-sealing, structural
wound-healing, and anti-herbivore defenses. In previous studies there
have been two chemical defense theories, phlorotannins being in
either generally high concentrations at all times that discourage
herbivores or a reactive response to grazing, stimulating an increased
production of phlorotannins that prevents further herbivory. But
Luder found that in grazer-preference experiments, herbivorous snails
preferred freshly wounded
Fucus distichus
over uninjured thalli, but as the macroalgae responded to being wounded
by accumulating greater concentrations of phlorotannins at
the wound area, the snails shifted their preference towards the
uninjured controls suggesting that phlorotannins might be
considered as an inducible chemical defense agent that deters
herbivores from
further eating while preventing infection by microbes such as
bacteria or fungi, showing that
phlorotannins
do indeed serve multi-purpose roles. This study also answered my own
question as to the role of secondary metabolites being able to deter
gastropods. They obviously do.
When local macroalgae were transplanted
(Mantyka 2007)
from their protected, shallow reef flat farther offshore onto the
nearby, deeper coral reef, only two of the twelve macroalgae species
(Galaxaura sp. and C. fastigiata)
showed any sign of deterring all herbivore fish species. It is worthy
to note that the coral reef selected contained a high diversity of
herbivore fish species without population reductions typical of
other reef locations, making the algae subject to naturally occurring
levels of grazing. The species of herbivore fish and their
diversity of grazing preferences along with the macroalgae being of
local species most likely played a large role in the herbivores being
able to control the majority of transplanted algae species, an example
of a predator and prey evolutionary arms race that has prevented algae
dominance on local coral reefs, naturally. When algae species are
introduced into areas that they are not naturally
found in, the local herbivores have not had the thousands of years in
which to develop counter measures against the algal
defenses allowing
the algae to grow uncontrollably as witnessed with the
Caulerpa spp. in the mediteranean.
In Mantyka's study, 19 fish species were examined for their
effectiveness at algae removal by counting the number of bites each
species took among all the algae species presented to them. Of the 19
species, six were found to represent 87% of the total standardized
bites. The six species were:
S. doliatus, C. microrhinos, S. canaliculatus, H. longiceps, Scarus rivulatus and
P. sexstriatus. Within the study, there was a distinct separation of
Sargassum sp. from all other macroalgae based on the grazing by
S. canaliculatus whose
feeding was primarily upon the
Sargassum sp. Also of note was
S. doliatus feeding primarily upon the
Hypnea sp. I am very interested in looking further into the local population levels of
S. canaliculatus here at Mactan Island as I have observed the
Sargassum spp.
slowly encroaching upon the coral reef areas over the last few years
with no obvious grazing being done upon this very invasive algae. Might the reintroduction of
S. canaliculatus aide the restoration of the coral reefs lost to this algae species?
Chemical defensive
measures produced by macroalgae are not fool proof. With thousands of
algal species and their diverse array of compounds, the equally diverse
herbivores have managed to evolve and counter many of the algal
defenses. What becomes apparent is that the chemical defenses do not
deter all herbivores, but instead reduces the number of herbivore species that
find specific algae species palatable down to a number to where the
algae has a fighting chance at survival by not being eaten by all species
of herbivores.
Many herbivores have also developed a
specific need for some of the chemicals produced by the algae as part of their
own life history, actively seeking out specific algae just for their
chemical content. Sea hares and some nudibranch species are a good
example of such needs, incorporating what was supposed to be a chemical
deterrent into their own bodies, stealing the compounds if you will, and putting
them to use for their own defense against predators. Certain amphipod
species have become so dependent upon specific algal compounds that
the absence of the compounds in their diet can cause a drop in amphipod
fertility.
Conclusion :
Having been blessed with the opportunity to observe this and
other marine habitats for a number of years and the freedom of time to
research what I have observed, I feel the need to stress the fact that
I have only been able to briefly touch upon each of the above
subjects simply due to their complexity and the number of
other
subject matters involved within any aspect of such complexity.
Pick a single subject and it is conceivable that one
could
make a career of its study. The vast diversity of species, their
multiple interactions, the processes in which they consume and produce
nutrients, the transportation methods of those nutrients, the slightest differences in climate, geomorphology
and
geochemistry all having profound effects that resonate back and forth
along the multiple pathways that extend from the land out to the
deepest of the oceans will ensure that we will remain ignorant for a
good long time to come.
All this and I
am only half
way 'there' as I "walk" these articles out to the coral reefs.
For
me to feel the need to state the above at this point is a testimonial
of the awe that only the oceans can instill, if one only
looks.
 My 20 gallon Macroalgae Refugium
My 20 gallon Macroalgae Refugium
Macroalgae Refugium Setup
With what we have learned of the macroalgae dominated reef flats,
a simple refugium dedicated to a variety of macroalgae
species will provide a very dynamic habitat that can only be of benefit
to a coral display aquarium. With the fauna and infauna having
been provided their natural habitats, they in turn will not only
process nutrients but in their breeding go towards feeding your corals
a live, natural diet of zooplankton. The macroalgae you select
will also respond to any nutrients and through their growth maintain a
naturally balanced aquarium system.
Preferably the aquarium to be used as the refugium should be as large
as possible as the macroalgae subtidal areas in relation to the coral
reefs are substantially larger, providing the nutrient poor conditions
that self limit the macroalgae from the coral reef while also providing
a great deal of food in the form of detritus and zooplankton.
Ideally the macroalgae refugium should be three to four times
larger than the coral display aquarium but given space constraints, a
refugium at least as large as the coral display aquarium would suffice.
The Sandbed 
Unlike the seagrasses meadows, the macroalgae subtidal areas are
comprised of a slightly larger grain size with fragments of coral
rubble mixed in. This type of sandbed material while able to trap
detritus and food particles better than a very fine grained
sand will provide the correct environment and food for a
number of sand infauna,
most notably the Copepods, Amphipods and Nematodes. A depth of at
least 2-3 inches will suffice in providing enough vertical space for
the infauna to live within. For more information about sandbeds
and their importance to the functionality of a reef aquarium system,
please see Dr. Ron Shimek's article on
How SandBeds Really Work.
Live Rock Substrate 
Usually thought of as simply a surface area for the macroalgae
to attach to, the use of base or cured live rock defeats the potential
of what the refugium can bring to the entire aquarium system. As such,
you will gain greater diversity of both fauna and flora through
the use of quality uncured live rock, landscaped in a way that allows
for maximum exposed surface area to the light. Simply laying flat
sections of live rock on top of the sandbed will replicate the same
features as found in the subtidal zones, but if there is ample space
within the larger refugiums, the stacking of a portion of the live rock
is acceptable as it will create cryptic areas that will further the
diversity of life within the refugium. If done so, water flow
into the cryptic areas will have to be provided with the use of a
small, low flow powerhead as such life forms require water flow in
order to thrive. Such flow should only be directed under the
stacked live rock and blocked from creating excessive water flow over
the open areas of the refugium where the macroalgae will grow.
This is an important consideration if one wishes to create the
very low flow conditions within the macroalgae mats that allow the
algae to perform nitrification and denitrification as happens naturally
in the subtidal zones.
Given that any refugium, regardless
of its size is a relatively small and confined space, it is very
important that some of the larger predatory or herbivore species of
animals that may hitch hike in on the live rock be removed. It
would be very counter-productive to have one or more crabs, mantis
shrimp or eunicid worms consume the other, more beneficial life forms
that also hitch hiked in. Adding herbivores or allowing hitch
hiking herbivores to remain also presents a problem as any such animals
can decimate the algae that is meant to grow and sequester nutrients
for removal and not simply be recycled back into the system having been
eaten by large herbivores such as snails or fish. The smaller
herbivores such as amphipods are to be encouraged as their grazing
is relatively small and unnoticeable while the amphipods themselves go
towards feeding the rest of the aquarium system.
If by
chance you obtain a variety of algae species growing upon the live
rock, you can expect one or more of the species to become dominate
while others fade away and disappear. This is to be expected and
is perfectly natural as those algae species that do thrive and dominate
are matching what your aquarium system is providing and is in need of
control. If you purposely add algae species, do not expect all or
any of them for that matter, to thrive or even survive. Given the vast
differences between the algae species, it will be a hit or miss
affair when trying to match species to a specific aquarium's
parameters, although one should try and take advantage of those algae
species that have known requirements. If one or more species
become dominant, be thankful
and appreciate the job it or they will do for your aquarium regardless
if the dominant species is "pretty" or not.
Macroalgae Selection 
Given their differing abilities, the selection of macroalgae
species to be kept can be tailored to suit the needs of an individual
aquarium system. Keeping with the natural theme of how I maintain
my aquarium system I have chosen to use the filamentous algae as
detailed in Part One of this article series for dealing with sudden
influxes of nitrogen and phosphorous as occurs immediately after the
addition of prepared foods to the system. The macroalgae species
that I keep in the refugium also respond to sudden inputs of nutrients
but are also capable of enduring the low nutrient levels between such
inputs. I have found that the
Phaetophyta and
Rhodophyta
species best serve this purpose due to their abilities to store
nitrogen which allows them to endure very low nutrient levels unlike
most of the
Chlorophyta
species that have very limited storage capacity making them vulnerable
to dieing off when nutrient levels approach the target levels that we
aim for when keeping corals. Additionally, the
Rhodophyta's
accessory pigments are best adapted towards lowered light intensity
allowing the use of inexpensive lighting systems and reducing the
electrical costs of running a reef aquarium system.
Keeping
the mat forming
Phaetophyta and bush-like
Rhodophyta creates the
required habitat for any number of epifaunal animals that through their
own actions will add to the natural nutrient dynamics of an aquarium
system, further reducing the need to add prepared foods while providing
the correct sized live foods that many corals require.
Water Parameters
With their mechanisms to deal with extremes in temperature and
irradiance levels, any of the tropical macroalgae will do well in a
tropical reef aquarium system that maintains normal reef parameters.
Maintaining a low flow through the refugium by simple bulk water
movement through the overflow system will best replicate the naturally
occurring low flows through the macroalgae subtidal zones allowing food
particles and detritus to settle out of the water being received from
the coral display aquarium, which will go towards feeding the multitudes of life forms that
will flourish in such an environment.
In addition to
providing a habitat and removing nutrients, the macroalgae will also be
of help in reducing the build up of metals within the system.
A review of the metal contents within any of the commercially
available dry salt mixes show that all brands do not even come
close to the metal content of natural salt water, such unnaturally high
levels provided by salt mixes could lead to the build up of dangerous
heavy metals within a reef aquarium system and since the macroalgae are
quite capable of sequestering large amounts of
such metals, their being trimmed and removed from the system are not
only exporting nutrients, but most likely a good
amount of the metals as well.
Lighting 
Given their ability to utilize varying degrees of light intensity
through the use of their accessory pigments, the macroalgae do not
require costly high intensity lighting systems and will do well
under energy efficient household compact fluorescent light bulbs
that require nothing more than a socket, wire and a plug with no need
for energy consuming, heat producing ballasts. Such light bulbs are
available in a 6,500 kelvin providing a more eye pleasing setting while
providing for the macroalgae needs.
I do not recommend that a planted refugium be kept on an extended
lighting period as the majority of algae require a dark period for
proper respiration and growth. However, the planted refugium can
be kept on an alternate light cycle with the coral display aquarium (12
hours on, 12 hours off) as this will help to keep the system oxygenated
and provide a more stable pH of the water.
Care & Maintenance
With
our addition of food for both corals and fish, the excess of nutrients
that most aquarium systems struggle with will provide more
than enough
nitrogen and phosphorous to maintain the macroalgae. With the
correct selection of species,
any drop in nutrient availability will not cause the loss of the
macroalgae but only a slowing of their growth, able to stand by and ready
to
take advantage of any nutrient increases, leaving little to be done in
the actual care of the macroalgae other than the possible need to
supplement bioavailable iron during periods of rapid growth. Any
brand of liquid, chelated iron available to our hobby will suffice if
you note an obvious yellowing of the macroalgae or a fading of its
normal coloration. With iron test kits available it is a simple
matter to maintain natural saltwater levels of .0034 ppm.
With the algae growing and the refugium starting to get crowded, it is at
this time that you should trim the algae and throw it away, although a
small part of it may be fed to fish herbivores to supplement their
diet, that is if the species of fish being kept find the macroalgae
that you keep to be palatable, if they ignore it or take only
exploratory bites then just throw it away.
Prior to trimming
the algae you should have a sufficient amount of new saltwater prepared
in order to do at least a 50% water change on the refugium as well as
replacing any carbon in use with new carbon. Once ready,
turn off all flow going into the refugium and trim the macroalgae
down by at least half its mass. This can be done with a pair of
scissors or by simply tearing or breaking the macroalgae with your
hands. Remove as much of the trimmed algae as possible but before
throwing it away, shake the algae at the surface of the water to
dislodge any animals that may be clinging to or living upon the
macroalgae that is about to be thrown away, then use a siphon hose
to remove 50% of the refugium's water while vacuuming up any remaining
small algal fragments.
The water and carbon change is
done since the act of tearing or cutting the macroalgae most likely
releases a good amount of the algae defensive compounds which if
allowed to become concentrated within an enclosed aquarium
system may put an unnecessary stress on other aquarium
inhabitants.
If you live near a coast, please do not flush
any viable algae fragments or the refugium's water into the drain
system of your home, doing so might introduce a foreign pest species
into the local habitat. Please sterilize any aquarium water with
bleach and let stand for an hour or two before pouring down any drain.
For large algae fragments, simply add to your household garbage
for disposal.
Conclusion :
Having observed and explored the complexity of the subtidal, macroalgae
dominated zones, it becomes obvious that in order to replicate a reef,
such a zone must be a part of any reef aquarium system. Having
even a fraction of the diversity of life as found in the subtidal areas
can and will provide for the needs of many of the larger animals that
we keep as pets while performing the tasks of nutrient control that
mechanical gadgets fail miserably at when compared against the algae
species that have had a great deal of time to evolve for just that
purpose. I think it is high time that this hobby gets back to
nature, which is after all the reason we keep such aquarium systems and
can only do so by incorporating the same systems that have worked so
well for the natural reefs. It is my opinion that a single
aquarium being used in an attempt to recreate a reef is nothing more
than an exercise in frustration and futility. Set up a refugium and
begin to enjoy a real reef system.
Related Reading
:
A Philippine Fringing Reef &
The
Reef Aquarium Part One - Land meets Ocean
Part Two - The Grass is Always Greener.... Part Four - The Tropical Sargassum Kelp
An Online Philippine Reef Tour
The Reef Aquarium Clean Up Crew Acknowledgments :
I would like to thank my wife Linda for her loving
support and understanding of my interests in all things marine. A
special thank you goes out to Eric Borneman for his generosity in
providing assistance with this article and in helping me to make sense
of tropical reefs. To Dr. Ron Shimek and Leslie Harris, thank you for
the many identifications made as well as teaching me a great deal about
marine biology and zoology.
References:
Andersson
M. et al. (2006) Different patterns of carotenoid composition and
photosynthesis acclimation in two tropical red algae. Marine
Biology (2006) 149: 653–665
Capone D.G. et al. (1992) Microbial nitrogen transformations in unconsolidated coral reef sediments. Mar Ecol Prog Ser 80: 75-88
Danovaro
R. et al. (2007) Trophic importance of subtidal metazoan meiofauna:
evidence from in situ exclusion experiments on soft and rocky
substrates. Mar Biol 152 : 339 – 350
Beach
K.S. et al. (1997) In vivo absorbance spectra and the
ecophysiology of reef macroalgae. Coral Reefs (1997) 16:21-28
Bertrand M. et al. (2005) Photosynthetic organisms and excess of metals. PHOTOSYNTHETICA 43 (3): 345-353
Boucher G. et al. (1998)
Contribution of soft-bottoms to the community
metabolism (primary production and calcification) of a barrier reef
flat. Journal of Experimental Marine Biology and Ecology,
225: 269–283
Engel S. et al. (2006) Antimicrobial
activities of extracts from tropical Atlantic marine plants against marine
pathogens and saprophytes. Marine
Biology, Volume
149, Number 5
Epstein
S.S. (1996) Microbial Food Webs in Marine Sediments. I. Trophic
Interactions and Grazing Rates in Two Tidal Flat Communities.
Microbe Ecol 34:188–198
Erickson
A.A. et al. (2206) Palatability of Macroalgae that Use Different
Types of Chemical Defenses Journal Chem Ecol Vol.32:
1883–1895
Gobler
C.J. et al. (1997) Release and bioavailability of elements
following viral lysis of a marine chrysophyte. Limnol
Oceanography 42:1492-1504
Gordon
R. et al. (2004) Effects of water motion on propagule release
from algae with complex life histories. Marine Biology 145:
21–29
Heil
C.A. et al. (2004) Benthic microalgae in coral reef sediments of
the southern Great Barrier Reef, Australia. Coral Reefs 23:
336–343
Hill
R.W. et al. (1998) Virus-mediated total release of
dimethylsulfoniopropionate from marine phytoplankton: a potential
climate process. Aquat Microb Ecol Vol, 14: 1-6
Hou X. et al. (1977) Determination of chemical species of
iodine in some seaweeds. The Science of the Total Environment 204:
215-221.
Israel
A. et al. (1999) Photosynthetic inorganic carbon utilization and
growth of Porphyra linearis (Rhodophyta). Journal of Applied
Phycology 11: 447–453
Jung V. et al. (2002) Comparison of the
wound-activated transformation of caulerpenyne by invasive and
noninvasive Caulerpa species of the Mediterranean. Journal of Chemical
Ecology, Vol. 28, No. 10.
Hay M.E. et al. (1988) Can tropical seaweeds reduce herbivory by growing at night? Diel patterns
of growth, nitrogen content, herbivory,
and chemical versus morphological defenses. Oecologia,
Volume
75, Number 2
Kain
J.M. et al. (1999) Photosynthetic and respiratory responses of
the agarophyte Gelidiella acerosa collected from tidepool, intertidal
and subtidal habitats. Hydrobiologia 398/399: 321–328
Kalita
T.L. (2003) Effect of Temperature and Illumination on Growth and
Reproduction of the Green Alga Ulva fenestrata. Russian Journal
of Marine Biology, Vol. 29, No. 5: 316–322
Kamer
K. et al. (2004) The relative importance of sediment and water
column supplies of nutrients to the growth and tissue nutrient content
of the green macroalga Enteromorpha intestinalis along an estuarine
resource gradient. Aquatic Ecology 38: 45–56
Khristoforova
N.K. et al. (1980) Mineral Composition of Seaweeds from Coral
Islands of the Pacific Ocean as a Function of Environmental Conditions.
Mar Ecol Prog Series Vol. 3: 25-29
Kladi
M. et al. (2004) Volatile halogenated metabolites from marine red
algae Phytochemistry Reviews 3: 337–366
Klenell
M. et al. (2004) Active carbon uptake in Laminaria digitata and
L. saccharina (Phaeophyta) is driven by a proton pump in the plasma
membrane. Hydrobiologia 514: 41–53
Krause-Jenson D. et
al. (1999) Oxygen and Nutrient Dynamics Within Mats of the Filamentous Macroalga
Chaetomorpha linum. Estuaries Vol. 22, No. 1, p. 31-38
Kristensen
E. (2000) Organic matter diagenesis at the oxic/anoxic interface
in coastal marine sediments, with emphasis on the role of burrowing
animals. Hydrobiologia 426: 1–24
Kubanek
J. et al. (2004) Ambiguous role of phlorotannins as chemical
defenses in the brown alga Fucus vesiculosus Mar Ecol Prog
Series Vol. 277: 79–93
Lapointe B.E. (1989) Macroalgal production and nutrient relations in oligotrophic areas of Florida Bay. Bull mar Sci 44: 312-323
Lapointe
B.E. et al.(1992) Nutrient availability to marine macroalgae in
siliciclastic versus carbonate-rich coastal waters. Estuaries 15: 75-82
Larned
S.T. et al. (1997) Nitrogen-limited growth in the coral reef
chlorophyte Dictyosphaeria cavernosa, and the effect of exposure to
sediment-derived nitrogen on growth. Mar Ecol Prog Ser 145: 95-108
Larned
S.T. (1998) Nitrogen- versus phosphorus-limited growth and sources of
nutrients for coral reef macroalgae Marine Biology 132:
409-421
Litter,
M.M. et al. (1984) Heterotrichy and survival strategies in the
red
algae Corallina officinalis L. Bot. Mar. 27, 37–44.
Liu
J. et al. (2000) Responses of the macroalga Gracilaria tenuistipitata
var. liui (Rhodophyta) to iron stress Journal of Applied
Phycology 12: 605–612
Luder
U. et al. (2004) Induction of phlorotannins in the brown
macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to
simulated herbivory: the first microscopic study. Planta
218: 928–937
MacIntyre
H.L. et al. (1996) Microphytobenthos: The Ecological Role of the
"Secret Garden" of Unvegetated, Shallow-Water Marine Habitats. I.
Distribution, Abundance
and Primary Production. Estuaries Vol. 19, No. 2A, p. 186-201
Mantyka
C.S. et al. (2007) Direct evaluation of macroalgal removal by
herbivorous coral reef fishes Coral Reefs Vol. 26:435–442
Meyer
K. D. et al. (1994) Effects of seaweed extracts and secondary
metabolites on feeding by the herbivorous surgeonfish Naso lituratus.
Coral Reefs 13:105–112.
Multer
H.G. (1992) New Roles for Halimeda Holdfasts. Proceedings
of the Seventh International Coral Reef Symposium, Guam, 1992, Vol. 2
Nacorda
H.E. et al. (1997) Structure and temporal dynamics of macroinfaunal
communities of a sandy reef flat in the northwestern Philippines.
Hydrobiologia 353: 91–106
Naim
O. (1993) Seasonal responses of a fringing reef community to
eutrophication (Reunion Island, Western Indian Ocean). Mar Ecol Prog
Ser 99: 137-151
Pederson
M.F. et al. (1997) Nutrient control of estuarine macroalgae:growth
strategy and the balance between nitrogen requirements and uptake.
Mar Ecol Prog Series Vol. 161: 155-163
Raven
J.A. et al. (1999) The role of trace metals in photosynthetic
electron transport in O2-evolving organisms. Photosynthesis
Research 60: 111–149
Raven
J.A. (2003) Inorganic carbon concentrating mechanisms in relation
to the biology of algae. Photosynthesis Research 77: 155–171
Rost B. et al. (2006) Carbon acquisition of marine phytoplankton: effect of photoperiod length.
American Society of Limnology and Oceanography Vol 51: 12-20.
Rozan,
T. F. et al. (2002) Iron-sulphur-phosphorus cycling in the
sediments of a shallow coastal bay: implications for sediment nutrient
release and benthic macroalgal blooms. Limnology and Ocenaography 47:
1346–1354.
Snelgrove
P. (1998) The biodiversity of macrofaunal organisms in marine
sediments. Biodiversity and Conservation 7, 1123-1132
Thornton
D.C. (2004) Formation of transparent exopolymeric particles (TEP) from
macroalgal detritus, Mar Ecol Prog Series Vol. 282: 1–12
Troch
M. et al. (2006) Is diatom size selection by harpacticoid
copepods related to grazer body size? Journal of Experimental
Marine Biology and Ecology 332 1–11
Vides
L.C. (2002) Clonal architecture in marine macroalgae: ecological and
evolutionary perspectives. Evolutionary Ecology 15: 531–545
Villares
R. et al. (2005) Metal Levels in Estuarine Macrophytes:
Differences among Species. Estuaries Vol. 28, No. 6, p. 948~56
Walters
L.J. et al. (1994) Rapid rhizoid production in Halimeda discoidea
Decaisne (Chlorophyta, Caulerpales) fragments: a mechanism for survival
after separation from adult thalli. Mar. Biol. Ecol. 175,
105–120.
Wang
Q. (2007) Effects of circadian rhythms of fluctuating temperature
on growth and biochemical composition of Ulva pertusa.
Hydrobiologia 586:313–319.
Young
A.J. et al. (1997) The xanthophyll cycle and carotenoid-mediated
dissipation of excess excitation energy in photosynthesis Pure
&Appl. Chern., Vol. 69, No. 10, pp. 2125-2130.
© 2011 All Rights Reserved
Content
including photographs are copyright protected and may not be
used
or reproduced without written permission of the authors.






 The velocity of tidal currents are defined by local geography
determining the direction and speed at which the tides enter and exit
the reef flats. For the Camotes Sea the surrounding islands form
relatively narrow straits and channels with tidal flows
running parallel to the majority of the coast lines.
The velocity of tidal currents are defined by local geography
determining the direction and speed at which the tides enter and exit
the reef flats. For the Camotes Sea the surrounding islands form
relatively narrow straits and channels with tidal flows
running parallel to the majority of the coast lines. 

 The largest controlling factor for the
colonization and suvivorship of sediment infauna is the sediment
itself, its composition and sizes of material, be it calcium carbonate
or terrigenous
in source. Sand grain sizes also determines the
nutrient dynamics of the sediment and its suitability for habitation by
infauna, influencing community structure.
The largest controlling factor for the
colonization and suvivorship of sediment infauna is the sediment
itself, its composition and sizes of material, be it calcium carbonate
or terrigenous
in source. Sand grain sizes also determines the
nutrient dynamics of the sediment and its suitability for habitation by
infauna, influencing community structure. 



 Within the patchwork of unshaded and so called
unvegetated sediments found throughout the macroalgae habitat live
mixed communities of microscopic pennate
diatoms, dinoflagellates, and cyanobacteria forming sediment
communities occupying several microhabitats within, on and near the
sediments suggesting that they have multiple functions and are
very important contributors to primary production.
Within the patchwork of unshaded and so called
unvegetated sediments found throughout the macroalgae habitat live
mixed communities of microscopic pennate
diatoms, dinoflagellates, and cyanobacteria forming sediment
communities occupying several microhabitats within, on and near the
sediments suggesting that they have multiple functions and are
very important contributors to primary production. These functional autotrophs and mixotrophs can be found living epiphyticaly on the sand grains and macroalgae while epipelic
groups of diatoms and dinoflagellates migrate within the sediments
acting as food for sediment infauna, sediment stabilizers that help to
prevent particle suspension and as regulators of nutrient exchange
between the sediments and the water column (Heil 2004).
These functional autotrophs and mixotrophs can be found living epiphyticaly on the sand grains and macroalgae while epipelic
groups of diatoms and dinoflagellates migrate within the sediments
acting as food for sediment infauna, sediment stabilizers that help to
prevent particle suspension and as regulators of nutrient exchange
between the sediments and the water column (Heil 2004). 
 It
may be tempting to overlook these microscopic producers and
focus solely on the larger macroalgae but given the profound effect
that so many tiny individuals have upon sediment nutrient
dynamics and their large contribution to primary production (up to
50%), they must be taken into account and appreciated when studying any
marine habitat that has exposed sediments and sufficient light
intensity to stimulate photosynthesis. The volumes of quality
food produced by the microphytobenthos forms the basis of many marine
food webs. They are truly a wonder.
It
may be tempting to overlook these microscopic producers and
focus solely on the larger macroalgae but given the profound effect
that so many tiny individuals have upon sediment nutrient
dynamics and their large contribution to primary production (up to
50%), they must be taken into account and appreciated when studying any
marine habitat that has exposed sediments and sufficient light
intensity to stimulate photosynthesis. The volumes of quality
food produced by the microphytobenthos forms the basis of many marine
food webs. They are truly a wonder. Most of the ocean's bottom is covered in sediments, making
the organisms that live within the sediments the largest faunal group
on Earth, sampled areas have led to estimates of infaunal species
numbers ranging from 500,000 up to 10,000,000, most of which have yet
to be described by science (Snelgrove 1998).
Most of the ocean's bottom is covered in sediments, making
the organisms that live within the sediments the largest faunal group
on Earth, sampled areas have led to estimates of infaunal species
numbers ranging from 500,000 up to 10,000,000, most of which have yet
to be described by science (Snelgrove 1998). Nematodes, by far the most abundant of the meiofauna regardless
of sediment conditions, found in both course grained and fine grained,
silted sediments comprising 22-84% of the meiofauna community
structure. With an estimated one million species and only a few
hundred actually known, there is still a great deal to learn of these
worms. Despite sharing basic morphology, nematodes occupy
numerous roles in sediments, feeding on bacteria, on algae or on
both, they eat detritus and possibly dissolved organic matter while quite a
few are predators, feeding on other nematodes, oligochaetes and
polychaetes. Their diversity in feeding reflects their species
diversity making the number of nematode species in most
habitats much higher than that of any
Nematodes, by far the most abundant of the meiofauna regardless
of sediment conditions, found in both course grained and fine grained,
silted sediments comprising 22-84% of the meiofauna community
structure. With an estimated one million species and only a few
hundred actually known, there is still a great deal to learn of these
worms. Despite sharing basic morphology, nematodes occupy
numerous roles in sediments, feeding on bacteria, on algae or on
both, they eat detritus and possibly dissolved organic matter while quite a
few are predators, feeding on other nematodes, oligochaetes and
polychaetes. Their diversity in feeding reflects their species
diversity making the number of nematode species in most
habitats much higher than that of any  Harpacticoids are found to be restricted to the larger grained
sediments comprising 30-37% of the meiofauna community structure but
only 8-12% in fine grained silted sediments due to the lowered oxygen
content of such sediments. Harpacticoid copepods are known to eat
a variety of foods, including bacteria, algae, and detritus but
seem to prefer diatoms with different copepod species consuming a
preferred diatom size in relation to their own size (Troch 2006).
Second only to the nematodes in biomass and the transferring
of nutrients garnered from microphytobenthos, the copepods are a
vital component of the benthic food web.
Harpacticoids are found to be restricted to the larger grained
sediments comprising 30-37% of the meiofauna community structure but
only 8-12% in fine grained silted sediments due to the lowered oxygen
content of such sediments. Harpacticoid copepods are known to eat
a variety of foods, including bacteria, algae, and detritus but
seem to prefer diatoms with different copepod species consuming a
preferred diatom size in relation to their own size (Troch 2006).
Second only to the nematodes in biomass and the transferring
of nutrients garnered from microphytobenthos, the copepods are a
vital component of the benthic food web. Pseudomeiobenthos while a mixture of juvenile species, they do comprise
8-35% of the meiofauna community with their greatest numbers to be
found in fine grained silty sediments. While the
pseudomeiobenthos also make up a large percentage of the meiofauna,
they do so not by single species population densities but by there
being many species in combination, juvenile Polychaetes being the
most numerous. Bivalves make up the second largest group to be
found.
Pseudomeiobenthos while a mixture of juvenile species, they do comprise
8-35% of the meiofauna community with their greatest numbers to be
found in fine grained silty sediments. While the
pseudomeiobenthos also make up a large percentage of the meiofauna,
they do so not by single species population densities but by there
being many species in combination, juvenile Polychaetes being the
most numerous. Bivalves make up the second largest group to be
found.  Ostracods have their greatest density in fine grained silted
sediments comprising up to 10% of the meiofauna community while in
larger, course grained sediments their density level drops down to less
than 1%. One of the most successful crustacean groups
with 8000 living species with very diverse diets. The
majority of the species are found within sediments consuming organic
detritus, algae or other members of the meiofauna. Ostracods are
segmented crustaceans having a head, thorax and abdomen and while
bivalved, they are not clams. Only the head has the full
complement of limbs with five pairs of "hairy" legs and at least one
eye.
Ostracods have their greatest density in fine grained silted
sediments comprising up to 10% of the meiofauna community while in
larger, course grained sediments their density level drops down to less
than 1%. One of the most successful crustacean groups
with 8000 living species with very diverse diets. The
majority of the species are found within sediments consuming organic
detritus, algae or other members of the meiofauna. Ostracods are
segmented crustaceans having a head, thorax and abdomen and while
bivalved, they are not clams. Only the head has the full
complement of limbs with five pairs of "hairy" legs and at least one
eye.  A
species sampling (Nacorda 1997) performed
within the sediments of a reef flat offshore of Santiago
Island, The Philippines, encountered 98 taxa from
11 major groups. The majority of which were members of the Annelida.
The Polychaete order Phyllodocida containing 13 families represented
the most abundant order. The arthropods consisted of 20 crustacean taxa
(malacostracans and ostracods) and 2 chelicerate taxa (marine mites and
sea spiders). Gastropods, bivalves, and scaphopods (Dentaliidae) made
up the mollusks. Other groups encountered were the cnidarians
(young octocorals and hydroids) and echinoderms (small ophiuroids,
holothurians, and echinoids), flatworms (Turbellaria), chaetognaths
(the benthic genus Spadella), nemerteans, and sipunculids. Fish larvae
and cephalochordates (Branchiostoma sp.) represented the chordates.
A
species sampling (Nacorda 1997) performed
within the sediments of a reef flat offshore of Santiago
Island, The Philippines, encountered 98 taxa from
11 major groups. The majority of which were members of the Annelida.
The Polychaete order Phyllodocida containing 13 families represented
the most abundant order. The arthropods consisted of 20 crustacean taxa
(malacostracans and ostracods) and 2 chelicerate taxa (marine mites and
sea spiders). Gastropods, bivalves, and scaphopods (Dentaliidae) made
up the mollusks. Other groups encountered were the cnidarians
(young octocorals and hydroids) and echinoderms (small ophiuroids,
holothurians, and echinoids), flatworms (Turbellaria), chaetognaths
(the benthic genus Spadella), nemerteans, and sipunculids. Fish larvae
and cephalochordates (Branchiostoma sp.) represented the chordates.  Salinity had
the largest impact for most groups population levels during
the monsoonal wet season as shown in the population chart
compiled
from Nacorda's study (see figure #1). With increased
rainfall,
the lowered salinity causes a drop in the macroinfauna's population
along with a shift towards diminished diversity among the groups
sampled. It is unclear what affects this temporary decline in
macroinfauna populations has upon the sediment's nutrient dynamics but
one could assume that a drop in population levels of any infaunal group
would reverberate up and down the food web.
Salinity had
the largest impact for most groups population levels during
the monsoonal wet season as shown in the population chart
compiled
from Nacorda's study (see figure #1). With increased
rainfall,
the lowered salinity causes a drop in the macroinfauna's population
along with a shift towards diminished diversity among the groups
sampled. It is unclear what affects this temporary decline in
macroinfauna populations has upon the sediment's nutrient dynamics but
one could assume that a drop in population levels of any infaunal group
would reverberate up and down the food web. 
 The mixture of coral rubble fragments within the
carbonate sediment provides macroinfauna stabilizing structures in
which to form extensive burrows. The soft sediments provide habitat for
multitudes of macroinfauna that are capable of creating tube
linings and microinfauna that simply live between the grains of the
sediment.
The mixture of coral rubble fragments within the
carbonate sediment provides macroinfauna stabilizing structures in
which to form extensive burrows. The soft sediments provide habitat for
multitudes of macroinfauna that are capable of creating tube
linings and microinfauna that simply live between the grains of the
sediment.  With the amount of coral
rubble
present and its weathered appearance, it is apparent that
decades
ago this area held substantial shallow water coral reefs. I can only
speculate that the shift from coral dominance to algae dominance was
the result of human development of the island resulting in increased
amounts of nutrients being washed into the ocean during the monsoons
and its heavy rainfall.
With the amount of coral
rubble
present and its weathered appearance, it is apparent that
decades
ago this area held substantial shallow water coral reefs. I can only
speculate that the shift from coral dominance to algae dominance was
the result of human development of the island resulting in increased
amounts of nutrients being washed into the ocean during the monsoons
and its heavy rainfall. As a stable structure, the calcium carbonate rocks
that are found in abundance within the subtidal zone create a
sheltered habitat for many animals while providing a solid substrate
for the macroalgae to grow upon, providing yet another habitat that in
combination with the rock makes for a very diverse community of both
algae and animal species.
As a stable structure, the calcium carbonate rocks
that are found in abundance within the subtidal zone create a
sheltered habitat for many animals while providing a solid substrate
for the macroalgae to grow upon, providing yet another habitat that in
combination with the rock makes for a very diverse community of both
algae and animal species.  The Eunicids are the largest and most numerous of the predatory
worm species comprising 60% of the worm species that I find living
within the rock's tunnels, of which they never fully exit from yet extend
outwards from their tunnel onto the sediment or up into the
macroalgae in search of food. They are also capable of extending
their range or reach by creating tunnels from small rock fragments that
they "glue" together in order to move farther afield while remaining
under cover and protected from predators. In doing so, the
Eunicids are credited with creating or extending coral reefs as the
corals find such structures suitable to settle upon and create new
coral colonys.
The Eunicids are the largest and most numerous of the predatory
worm species comprising 60% of the worm species that I find living
within the rock's tunnels, of which they never fully exit from yet extend
outwards from their tunnel onto the sediment or up into the
macroalgae in search of food. They are also capable of extending
their range or reach by creating tunnels from small rock fragments that
they "glue" together in order to move farther afield while remaining
under cover and protected from predators. In doing so, the
Eunicids are credited with creating or extending coral reefs as the
corals find such structures suitable to settle upon and create new
coral colonys.  The
Amphinoids are another common predator and scavenger within and amongst
the rock structures appearing to be much more mobile traveling between
rocks in search of their food. This apparent freedom to roam
openly is most likely due to their being very well defended by
their calcareous
setae that are filled with poisonous secretions, hence their commonly
being called the fire worms.
The
Amphinoids are another common predator and scavenger within and amongst
the rock structures appearing to be much more mobile traveling between
rocks in search of their food. This apparent freedom to roam
openly is most likely due to their being very well defended by
their calcareous
setae that are filled with poisonous secretions, hence their commonly
being called the fire worms.  The Cirratulids are deposit feeders which gather food by
means of their palps. They are sluggish worms with varied life
styles, some species bury themselves
below the surface of sea bottoms leaving only their gills and palps
visible. Some are free-living and inhabit tubes, while others are
capable of burrowing through corals, shell or rock. They occur
both in shallow and deep sea areas.
The Cirratulids are deposit feeders which gather food by
means of their palps. They are sluggish worms with varied life
styles, some species bury themselves
below the surface of sea bottoms leaving only their gills and palps
visible. Some are free-living and inhabit tubes, while others are
capable of burrowing through corals, shell or rock. They occur
both in shallow and deep sea areas.  The Thalassinidean shrimp account for the creation of many of
the larger burrows that run through the rock remaining very cryptic
only venturing to the openings of their burrows to feed upon detritus.
Their constant movement of the water and their respiration
account for a large portion of the nutrient processing as their
oxygenated burrows provide for aerobic bacteria.
The Thalassinidean shrimp account for the creation of many of
the larger burrows that run through the rock remaining very cryptic
only venturing to the openings of their burrows to feed upon detritus.
Their constant movement of the water and their respiration
account for a large portion of the nutrient processing as their
oxygenated burrows provide for aerobic bacteria.  With the vast amount of vegetative matter to be found in the
subtidal zone, there are vast amounts of herbivore species taking
advantage of such bounty while others find the vegetative cover and its
taller structures a suitable substrate on which to settle and grow,
however temporary such substrate may be. Of course where there
are herbivores, there are the predators.
With the vast amount of vegetative matter to be found in the
subtidal zone, there are vast amounts of herbivore species taking
advantage of such bounty while others find the vegetative cover and its
taller structures a suitable substrate on which to settle and grow,
however temporary such substrate may be. Of course where there
are herbivores, there are the predators. The Sea Spiders are a large group containing many species, some
much larger than others but all are predatory, stalking their prey
amongst the macroalgae.
The Sea Spiders are a large group containing many species, some
much larger than others but all are predatory, stalking their prey
amongst the macroalgae.  Snails make up the largest percentage of herbivores found
amongst the macroalgae covered rock substrates with a very large
diversity of species, from the thumb nail sized cowrys to the four
inch long abalones. Predatory Whelk species are also very common
as they seek out and feed upon the herbivore species.
Snails make up the largest percentage of herbivores found
amongst the macroalgae covered rock substrates with a very large
diversity of species, from the thumb nail sized cowrys to the four
inch long abalones. Predatory Whelk species are also very common
as they seek out and feed upon the herbivore species.  Chlorophyta -
Unlike the red and brown macroalgae, most green macroalgae are found in
freshwater or terrestrial environments. Of the roughly 8,000 species,
only 10% are marine. However, the species of green
macroalgae that are found in the marine environment can be very
abundant by sheer biomass.
Chlorophyta -
Unlike the red and brown macroalgae, most green macroalgae are found in
freshwater or terrestrial environments. Of the roughly 8,000 species,
only 10% are marine. However, the species of green
macroalgae that are found in the marine environment can be very
abundant by sheer biomass. Rhodophyta -
With roughly 800 genera and 5,200 species there are
more red
macroalgae species in the oceans than there are of green and brown
macroalgae
combined. Most species are red or pink in color, the result of red
pigments called phycobilins that mask the green color of the
chlorophyll in their cells. Most red macroalgae are marine and live in
shallow-water environments.
Rhodophyta -
With roughly 800 genera and 5,200 species there are
more red
macroalgae species in the oceans than there are of green and brown
macroalgae
combined. Most species are red or pink in color, the result of red
pigments called phycobilins that mask the green color of the
chlorophyll in their cells. Most red macroalgae are marine and live in
shallow-water environments. Phaeophyta -
The brown macroalgae usually varies from olive green to dark
brown in
coloration. Their coloration is derived from an abundance of
yellow-brown fucoxanthin pigments which masks the green color of the
chlorophyll in their cells.
Phaeophyta -
The brown macroalgae usually varies from olive green to dark
brown in
coloration. Their coloration is derived from an abundance of
yellow-brown fucoxanthin pigments which masks the green color of the
chlorophyll in their cells.
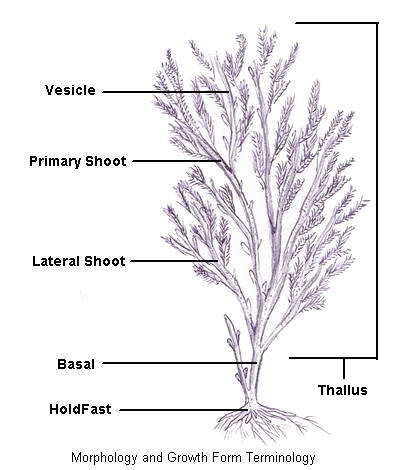


 The
Modules - Building blocks of the algae.
The
Modules - Building blocks of the algae.
 Its all about meiosis, a two-stage type of cell division in sexually reproducing organisms that results
in the development of sperm and egg cells. In meiosis, a diploid cell divides
to produce four haploid cells , each with half the original chromosome content.
In organisms with a diploid life cycle, the products of meiosis are called gametes. In organisms with an alternation of generations, the products of
meiosis are called spores.
Its all about meiosis, a two-stage type of cell division in sexually reproducing organisms that results
in the development of sperm and egg cells. In meiosis, a diploid cell divides
to produce four haploid cells , each with half the original chromosome content.
In organisms with a diploid life cycle, the products of meiosis are called gametes. In organisms with an alternation of generations, the products of
meiosis are called spores.






 Unlike the seagrasses meadows, the macroalgae subtidal areas are
comprised of a slightly larger grain size with fragments of coral
rubble mixed in. This type of sandbed material while able to trap
detritus and food particles better than a very fine grained
sand will provide the correct environment and food for a
number of sand infauna,
most notably the Copepods, Amphipods and Nematodes. A depth of at
least 2-3 inches will suffice in providing enough vertical space for
the infauna to live within. For more information about sandbeds
and their importance to the functionality of a reef aquarium system,
please see Dr. Ron Shimek's article on How SandBeds Really Work.
Unlike the seagrasses meadows, the macroalgae subtidal areas are
comprised of a slightly larger grain size with fragments of coral
rubble mixed in. This type of sandbed material while able to trap
detritus and food particles better than a very fine grained
sand will provide the correct environment and food for a
number of sand infauna,
most notably the Copepods, Amphipods and Nematodes. A depth of at
least 2-3 inches will suffice in providing enough vertical space for
the infauna to live within. For more information about sandbeds
and their importance to the functionality of a reef aquarium system,
please see Dr. Ron Shimek's article on How SandBeds Really Work.  Usually thought of as simply a surface area for the macroalgae
to attach to, the use of base or cured live rock defeats the potential
of what the refugium can bring to the entire aquarium system. As such,
you will gain greater diversity of both fauna and flora through
the use of quality uncured live rock, landscaped in a way that allows
for maximum exposed surface area to the light. Simply laying flat
sections of live rock on top of the sandbed will replicate the same
features as found in the subtidal zones, but if there is ample space
within the larger refugiums, the stacking of a portion of the live rock
is acceptable as it will create cryptic areas that will further the
diversity of life within the refugium. If done so, water flow
into the cryptic areas will have to be provided with the use of a
small, low flow powerhead as such life forms require water flow in
order to thrive. Such flow should only be directed under the
stacked live rock and blocked from creating excessive water flow over
the open areas of the refugium where the macroalgae will grow.
This is an important consideration if one wishes to create the
very low flow conditions within the macroalgae mats that allow the
algae to perform nitrification and denitrification as happens naturally
in the subtidal zones.
Usually thought of as simply a surface area for the macroalgae
to attach to, the use of base or cured live rock defeats the potential
of what the refugium can bring to the entire aquarium system. As such,
you will gain greater diversity of both fauna and flora through
the use of quality uncured live rock, landscaped in a way that allows
for maximum exposed surface area to the light. Simply laying flat
sections of live rock on top of the sandbed will replicate the same
features as found in the subtidal zones, but if there is ample space
within the larger refugiums, the stacking of a portion of the live rock
is acceptable as it will create cryptic areas that will further the
diversity of life within the refugium. If done so, water flow
into the cryptic areas will have to be provided with the use of a
small, low flow powerhead as such life forms require water flow in
order to thrive. Such flow should only be directed under the
stacked live rock and blocked from creating excessive water flow over
the open areas of the refugium where the macroalgae will grow.
This is an important consideration if one wishes to create the
very low flow conditions within the macroalgae mats that allow the
algae to perform nitrification and denitrification as happens naturally
in the subtidal zones.  Given their differing abilities, the selection of macroalgae
species to be kept can be tailored to suit the needs of an individual
aquarium system. Keeping with the natural theme of how I maintain
my aquarium system I have chosen to use the filamentous algae as
detailed in Part One of this article series for dealing with sudden
influxes of nitrogen and phosphorous as occurs immediately after the
addition of prepared foods to the system. The macroalgae species
that I keep in the refugium also respond to sudden inputs of nutrients
but are also capable of enduring the low nutrient levels between such
inputs. I have found that the Phaetophyta and Rhodophyta
species best serve this purpose due to their abilities to store
nitrogen which allows them to endure very low nutrient levels unlike
most of the Chlorophyta
species that have very limited storage capacity making them vulnerable
to dieing off when nutrient levels approach the target levels that we
aim for when keeping corals. Additionally, the Rhodophyta's
accessory pigments are best adapted towards lowered light intensity
allowing the use of inexpensive lighting systems and reducing the
electrical costs of running a reef aquarium system.
Given their differing abilities, the selection of macroalgae
species to be kept can be tailored to suit the needs of an individual
aquarium system. Keeping with the natural theme of how I maintain
my aquarium system I have chosen to use the filamentous algae as
detailed in Part One of this article series for dealing with sudden
influxes of nitrogen and phosphorous as occurs immediately after the
addition of prepared foods to the system. The macroalgae species
that I keep in the refugium also respond to sudden inputs of nutrients
but are also capable of enduring the low nutrient levels between such
inputs. I have found that the Phaetophyta and Rhodophyta
species best serve this purpose due to their abilities to store
nitrogen which allows them to endure very low nutrient levels unlike
most of the Chlorophyta
species that have very limited storage capacity making them vulnerable
to dieing off when nutrient levels approach the target levels that we
aim for when keeping corals. Additionally, the Rhodophyta's
accessory pigments are best adapted towards lowered light intensity
allowing the use of inexpensive lighting systems and reducing the
electrical costs of running a reef aquarium system.  Given their ability to utilize varying degrees of light intensity
through the use of their accessory pigments, the macroalgae do not
require costly high intensity lighting systems and will do well
under energy efficient household compact fluorescent light bulbs
that require nothing more than a socket, wire and a plug with no need
for energy consuming, heat producing ballasts. Such light bulbs are
available in a 6,500 kelvin providing a more eye pleasing setting while
providing for the macroalgae needs.
Given their ability to utilize varying degrees of light intensity
through the use of their accessory pigments, the macroalgae do not
require costly high intensity lighting systems and will do well
under energy efficient household compact fluorescent light bulbs
that require nothing more than a socket, wire and a plug with no need
for energy consuming, heat producing ballasts. Such light bulbs are
available in a 6,500 kelvin providing a more eye pleasing setting while
providing for the macroalgae needs. 