Having observed
the fringing reefs found near our home on
Mactan Island since 2004, We have become much more aware of not only how
the various habitats work in conjunction with each other, but also what
threatens
the balance that each of them brings to the coral reef equation. By
taking apart that equation, We hope to show how we can recreate the
reef's
solutions to functionality and apply them to reef aquariums, thus
creating more realistic, and
functional coral reefs within the glass boxes that we like to call our
reef aquariums. This series of articles will examine a Philippine
fringing reef's habitats and how we can apply each of those habitats to
our reef aquarium systems with the intention of creating a more
holistic and functional captive ecosystem that provides multiple
habitats each with their own unique populations that in combination
will provide the means to further the hobby towards establishing
aquarium systems that more accurately represent the meaning of the word
"reef."
Part Four : The Tropical Kelp
Previously in this
article series we explored the shoreline, a seagrass meadow and the
macroalgae dominated reef flat. This article will examine yet
another complex marine habitat, comprised of a few macroalgae species
commonly called kelp that dominate the shallow fore-reef and its substrate environment.
The Benthic Kelp Habitat The genus
Sargassum is distributed worldwide and forms dense monospecific or mixed stands in
sublittoral zones. Several species of
Sargassum
form underwater forests that provide habitat and spawning grounds to
marine invertebrates and fish, thus playing an important role as a
primary producers.
Having observed the local kelp beds for a number of years now, I have
been concerned that with the beds being located very close to the coral
reefs that I was witnessing a phase shift from hard coral dominance to
algae dominance. With the reefs being nearshore there is always
the concern that nutrient and particle run-off from man-made sources
and activities could quickly cause such a phase shift. However,
over the last few years it appears that for now, the algae and
seagrass beds are stable in that they do not appear to be encroaching
upon coral reefs yet I am fully aware that there is a delicate balance
that if tipped ever so slightly, could change the entire coastline's
habitats.
By having explored the dynamics involved
with the many algal and seagrass habitats, it is my hope that both you
and I have gained a greater appreciation and understanding that algae
and seagrass habitats perform a vital protective and nutritional function
for the coral reefs while at the same time posing a potential threat to
the reefs. In this last of the habitats prior to the coral reef,
the fine tuned balance between nutrient levels, light intensity,
habitat structure, herbivores and many other subtle variants becomes
even more critical and more easily upset as this habitat is the coral's
last defense against shore-based influences while at the same time
being under ever present and nearby threat of being over-run.
(Figure 1) Aerial view of
Mactan Island's exposed reef flats
As shown in Figure 1 there are distinct zones in which the various
habitats exist. For the kelp species that dominate the zone
between the macroalgal fields and the coral reef, it is the water
depth, canopy formation, substrate composition and most importantly, the lack of herbivores that allows the kelp
to remain dominate. The canopy formed by the kelp structures the
foliose algal assemblage by modifying the light environment
and this effect limits most other foliose species that are unable
to photoacclimate to the reduced light intensity under the much taller
kelp species. Water depth is also a factor in reducing light
intensity as well as restricting the kelp. In the frondose
macroalgal fields the low tides bring water depths to near exposure
levels which the kelp appears unable to endure along with greater
light intensity that does allow the foliose algae to compete against
the kelp as epiphytic growth.
However, as the kelp canopy lies relatively
close to the substratum, it not only reduces sub-canopy light levels
but also scours the substratum as the kelp is moved about in the
stronger water currents of its zone and hence some of the
effect on the understorey assemblage could also be explained by
thallus scour rather than canopy shading alone
(Toohey 2004).
Thallus
scour may also play an important indirect role in structuring the
foliose algal assemblage by modifying the distribution and activity of
invertebrates
(Velimirov 1979). If thallus scour does reduce the presence of
sessile invertebrates underneath the canopy, this may leave more primary space to be occupied
by
foliose algae; but if thallus scour reduces the distribution of or the
feeding activities of grazers such as sea urchins, then this
effect may be related to the recruitment of the kelp population
than to the species composition of the foliose algal assemblage.
Additionally, my own study on
the translocation of sea urchins as
a means to control kelp showed that the mobile sea urchins were unable
to move between established kelp and were unwilling to remain out of
contact with the substrate in order to climb up and over the kelp.
This behavior by the sea urchins would obviously limit their
grazing and give yet another advantage to the kelp as their being
grazed upon when colonizing substrates would be quickly reduced when
the juvenile kelp reaches but a few centimeters in length.
Weather Patterns
- A
seasonal nature.
With two very distinct seasons, a dry cloudless period that runs from
November to May and a wet cloudy monsoonal period that runs from June
to October, the variable effects upon shallow water habitats can be
profound.
The dry season
and its clear sunny days bring
surface water temperatures to their highest yearly average and
available dissolved nutrients to their lowest concentrations.
With the lack of rainfall providing terrestrial runoff and
very
little disturbance during the storm free dry season, the kelp
canopy being at the outer edge of the reef flat does not appear to be greatly affected as
the kelp receives little dissolved nutrients to begin with, having had any shore based nutrient
run-off first pass through the macroalgae and seagrass habitats.
The wet season and its cloudy nature brings surface water temperatures
down to the lowest yearly average while washing nutrient loaded run-off
into the ocean. Frequent storm driven waves suspends
organic
matter, shifts sediments and sets epiphytic macroalgae and kelp zygotes adrift. In
particularly heavy rain falls, the local salinity level may become near
brackish having a large impact on the fauna diversity and population
levels, temporarily altering the nutrient dynamics of the entire region.
- Life in the fast lane.
With
the force that water can bring upon a surface area, it is not
surprising that water velocity rates have very profound effects on local
species determining their distribution, community structure, nutrient
dynamics, availability of light and their morphology.
In the
protected reef flats, tidal flows are the primary source of water
velocity over
and through the kelp habitat on a daily basis while wind driven
wave action is a relatively rare occurance acting as a short lived
disturbance that the kelp in shallower areas recover from and in fact
may actually
benefit from, as viable algal fragments are torn away allowing the
dispersal of the species within and out of the local area.
The wave and wind action creates large floating mats of kelp that
usually drift farther out to sea forming a living, surface floating
shelter that many fish and invertebrate species take refuge within.
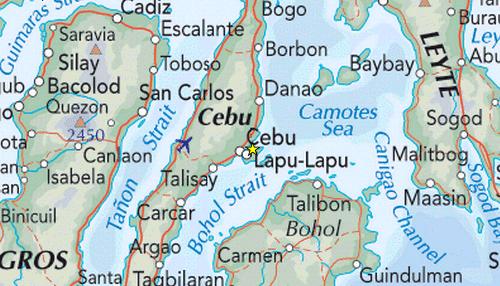
The velocity of tidal currents are defined by local geography
determining the direction and speed at which the tides enter and exit
the reef flats. For the Camotes Sea the surrounding islands form
relatively narrow straits and channels with tidal flows
running parallel to the majority of the coast lines.
For the kelp communities located on the edge of the shallow reef flats,
the parallel direction of the tidal flows (as shown below)
encounter obstacles that reduce the force of the flows. The
primary force reduction occurs when the flow is directed upwards as it
enters much shallower water depths and strikes the many large boulders
and coral outcroppings creating turbulence within the flow.
Once
past the coral reef and its barriers, the reduced flow enters the
deeper edge of the reef flat and its surrounding band of
kelp species
that extend their relatively large, wide blades
upwards to the surface creating a living wall for the water flow to run
up against.
Wind driven waves are a rare occurance on the reef flats as the
surrounding large islands shelter the straits leaving the tidal flows
as the only consistent means of water motion. High velocity rates
are very important to the overall health of any reef as the speed at
which water moves over the reefs have profound effects on
dissolved nutrient uptake rates
(Atkinson 1992),
species dispersal, food availability and gas exchanges while helping to
clear the reefs of smothering particulates and sediments. In
Atkinson's study using large flumes housing differing reef communities,
it was found that phosphate uptake rates increased in relation to water
velocity rates. The faster the water moved over the reef, the
faster the uptake rates. While the mechanism of enhanced uptake is
unknown, the study showed a strong, positive correlation with
water velocity.

Having observed the daily, weekly and monthly tidal rhythms for a
number of years now it became apparent that the tides follow a biweekly
cycle between rapid differences in the highs and lows and a much
gentler, expanded time difference between the high and low tides. During the
weeks that the tides are at their highest and lowest, it is by the
sheer bulk mass of 1.5 meters of water depth coming into and draining
out of the reef flats that the velocity blocking structures are
overcome, allowing the exchange of water to occur up to and including
the shallow shoreline areas. However, the bulk movement of the
water can only be described as being
gentle in comparison to the velocities that the fringing
coral reefs are subjected to. These weekly tidal differences also
determine when we go scuba diving on the fringing reefs as we
are
no swimming match against the stronger biweekly tides and restrict
ourselves to the macroalgae habitat and its gentle waters during these
week long tidal events.
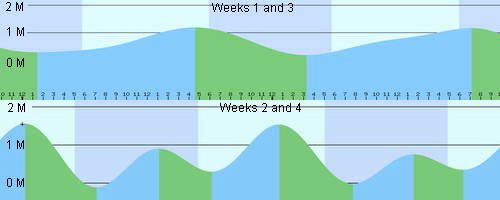 Average monthly tidal movements of Cebu Island, The Philippines
Average monthly tidal movements of Cebu Island, The Philippines
When the conditions are tide-dominated with slightly reduced velocities
as occurs on the subtidal edge of the reef flat, the
blades of the kelp are subjected to relatively high water flows,
creating a substrate themselves that many epiphytic animals take
advantage of and prosper by having a living substrate that is elevated
up into such water movement. In areas that are
wave-dominated, the
environment for the macroalgae is vastly different with much greater
forces being exerted upon them and allowing only those species capable
of withstanding the great mechanical stresses to survive, such as those
species with filamentous and crustouse morphologies. The larger,
frondose species would simply be torn away and driven onto shore.
Habitat Structure
The
kelp that dominate the outer edges of the reef flats are of those
species that anchor themselves through their holdfasts to stable,
unmoving substrates of which the outer reef flat provides in abundance
with large calcium carbonate boulders.
Although there are 8 species of Sargassum reported
(Ang, 1986) as being common within the Philippines, the two most prevelant species are
S. siliquosum and
S. paniculatum,
each
found in near equal abundance, often intertwined sharing the same
substrate. A number of other macroalgae species can also be found
in this zone yet appear to be incidental with far less growth and vigor
in comparison to the same species found closer to shore, making their
contributions to primary production as equally incidental.

While
the Sargassum kelp appears to be limited to specific zones that provide
it the proper growing and reproductive conditions, it does however
completely dominate its zones creating a monospecific habitat.
Such single species
habitats have been shown to be greatly reduced in diversity unlike
other habitats that have multiple algal species. With such low
diversity of habitat and algal food sources, herbivores and those
species that prey upon herbivores are also greatly reduced.
However, the three dimensional structure of the kelp provides a
dense, deep "thicket" that many juvenile fish find shelter and food
within, which in turn attracts a number of fish predators most of which
are comprised of the wrasse family that make short term, marauding
forays from the nearby coral reef into the kelp in search of food.

The most commonly found fish species amongst the kelp,
the filefish with its camouflaged colorations and patterns along with a thin, vertical body
form allows these species to move through the kelp stands nearly
invisible to both predators and prey. I have noted two color
forms, a brown (as shown) and a mottled green, the previous found
exclusively amongst the kelp while the latter is commonly seen amongst
the macro algae and/or the zone between the macroalgae and kelp beds.

Seahorses are another common fish species found amongst the many
"hitching posts" that the kelp provides, that is, if one looks close
enough as they too are very well camouflaged to match their
surroundings. The seahorses relative, the pipefish, are also
found in large numbers as they too exploit the kelp structure for both
safety from predators and as a hunting ground.

The Scribbled Rabbitfish is one of the few herbivores which are
found in any numbers within and near the Sargassum beds. I have
observed on many occasions, large schools of both juveniles and adults
grazing on the kelp's epiphytic growth, keeping the kelp blades free of
sun-blocking growths yet do not appear to consume any kelp. This
species is most sought after by local fishermen who place green algae
baited traps and remove large numbers of these fish each year.
Such removal of a herbivore is usually thought of as being
detrimental yet I believe that this fish species actually helps the
Sargassum by keeping its blades free of other growth while doing no
damage to the Sargassum itself.
Sargassum Morphology
Many aspects of the morphology of benthic Sargassum, as well as most
other alga vary in response to physical factors such as light intensity
and water flow. The differences in morphology, which include
buoyancy, affect the potential persistence of macroalgae in habitats
characterized by different water flow regimes. In
areas of slow water flow, the kelp will develop gas-filled floats
called pneumatocysts which provide a positive buoyancy whereas fronds
in high water flows or wave-exposed sites either lack pneumatocysts or
have very small pneumatocysts allowing for negative buoyancy. The
hydrodynamic forces experienced by an alga can be affected by the
alga's size, shape and the way it deforms in moving water.
(Stewart 2006).
The larger the algae, the higher the forces placed upon it. The
extent to which an algae can adapt its morphology depends upon its
shape and material properties. Thin, flexible fronds can be streamlined
much more easily than stiff, bushy fronds. But regardless of the
conditions, either high or low flow rate velocities, any adaptations
are meant for the alga's day to day survival and not meant to withstand
infrequent events such as storms, which will detach and set adrift the
algae regardless of its morphology.

The Anatomy of a Sargassum spp.
Sargassum is a well represented genus in tropical and warm temperate
regions throughout the world. The propagule of Sargassum is a
developing zygote. Sargassum expels eggs in a number of pulses over a
few days, loosely associated with the full or new moon The
eggs remain attached to the receptacles and the zygotes develop for at
least 24 to 48 hours before being released. Although most receptacles
synchronously release eggs, there is a highly variable number of
conceptacles on each receptacle producing eggs, and the time of release
of zygotes is variable.
Even with strong tidal currents most
propagules appear to settle out of the water column within meters of
parent algae and the greatest distances that propagules have been
recorded from their source was 1.7 km.
(Kendrick 1991).
This fact explains to me how kelp is able to fully dominate and
smother entire regions by just the sheer number of settling zygotes.
This is borne out within Deysher & Norton's 1982 study
where they seeded areas with propagules of
S. muticum from
transplanted, reproducing adults and found very dense recruitment
within 1 m of the parents and sparse recruitment up to 3 m away and
only a few recruits up to 30 m away. Even though the density of
propagules settling much further away from parents is small, successful
recruitment may still occur over much greater distances. Over
such distances, it has been proposed that drifting fragments of kelp
thalli is a more likely, or more successful mechanism for long term
dispersal, as can be said for any of the macro algae.

Tropical Sargassum kelp follow a yearly cycle that is characterized by
the presence of a slow growth phase, a rapid growth phase, and a
reproductive phase that is followed by senescence and die back. I
have observed that the die back period only occurs during those times
of the year when the tides are at their lowest, exposing the kelp to
dessication. The kelp also appears to grow slowest during the dry
season which may be related to higher temperatures as the kelp grows at
much faster rates during the cooler, wet season. In addition to
having its preferred cooler temperatures during the wet season, the
kelp also benefits from having land-based nutrient run-off provide an
increase in dissolved nutrients needed for growth and reproduction.
Temperature and irradiance are very
important factors influencing the growth of germlings and adults
in Sargassum species providing another insight as to local
environmental conditions required of this alga. Temperature
appears to be the greatest limiting factor as Choi's 2007 study
revealed near 100 percent fatality of Sargassum germlings and adult
alga at 30° C. A temperature level quite often obtained in
shallower, near shore areas that favor sea grasses and frondose
macroalgae species that are tolerant of, or capable of surviving such
temperature extremes.
Nutrient Dynamics
While
I do not have any long term data sets that would enable me to determine
if the macroalgae and seagrass beds are or have been expanding their
range locally, the few years that I have been observing such habitats
has not shown any such increases or decreases other than what is now
natural yearly cycles of growth and decline. That may all change
quickly though as the shoreline becomes further developed with
residential and industrial sources of nutrient and particle run-off.
Anytime mankind is involved, it is highly likely that rapid changes
will occur and is something that needs to be monitored closely. With
dissolved nutrient levels measured in minute differences having very
profound influences in the habitat's structure, any increases in those
levels will surely begin the phase shift towards complete algal
dominance of the coral reefs.
Disregarding the requirements
and effects of light intensity, water flow, temperature and herbivore
grazing, I view the shoreline turf algae, seagrass beds and macroalgae
beds as having removed a large part of the run-off nutrients leaving
little of said nutrients available to the Sargassum kelp species, which
by their very existence in what should be relatively nutrient poor
water hints at their having a requirement for a specific range of
nutrient levels or have the means to adjust to fluctuating levels.
My suspicions are borne out in Schaffelke's 1998 study of
nutrient limitations in Sargassum kelp which showed a direct
correlation between nutrient levels and the ability of the algae to
grow and expand its range. What I did find surprising is that the
Sargassum, while limited by nitrogen and phosphorous as was expected, the
algae also becomes limited by excessive amounts of nitrogen and
phosphorous.
In nutrient-limiting conditions
(Schaffelke 1998)
, the growth of Sargassum shoots first decreased, then stopped
altogether after fifteen days, indicating a nutrient storage capacity.
After twenty days the Sargassum begins to drop its blades and
experiences a die-back event yet is able to endure long periods of
nutrient poor conditions as occurs during the dry seasons. During
such conditions, the Sargassum endured with
slow-growing basal shoots which were found to contain much higher
nitrogen content than within the tissues of more distant blades.
I believe this observation can be explained by the Sargassum's
ability to absorb nutrients through its root-like holdfasts from
detrital matter that settles upon the holdfasts
(Schaffelke 2002), which this region
provides in great abundance.
In laboratory nutrient
enrichment studies, it was found that the equal combination of both
nitrogen and phosphorous produced the greatest growth of the plants if
such nutrient enhancement was provided in moderation (3 to 5
µM ammonium, 0.3 to 0.5 µM phosphate). The growth of newly
settled and young plants was also significantly faster in low and
moderate N and P enrichment than with the control specimens kept within
nonenriched seawater as would be expected of any algae.
Surprisingly though, when the equal enrichment of N and P was
increased the rates of growth were not increased indicating that the
Sargassum could not directly use the nutrients for rapid growth but
instead placed the nutrients in storage on which to draw from for
growth. This may be the plant's strategy to take advantage of
sudden, short lived nutrient increases as can occur during rain falls
by first ensuring that its storage capacity is fully utilized to allow
it to endure low nutrient periods instead of putting what may be
limited supplies of nutrients into rapid growth. In other words,
the Sargassum appears to top off its "tanks" first and only then starts
to use its full "tanks" for growth while keeping the "tanks" topped off
and will limit or cease its growth when nutrient levels drop, allowing
the plant to grow yet maintain full storage capacity for future use.
Growths rates were also observed to be limited by both N and P amounts.
Only when N and P are in equal proportions does the algae grow
its fastest. Reduce either N or P and growth rates slow.
This is telling as it may explain the recent problems many reef
areas are having with the expansion of Sargassum species into ranges
they were not previously found due to land based run-off of farm
fertilizers as such fertilizers usually contain a balanced N and P
formulation, making Sargassum an indicator species.


Due to its requirement of relatively deep water the Sargassum kelp
species are not something I would normally attempt to keep as I have
found that over the long term, my shallow refugium simply does not
provide the space for such vertical requirements allowing for a true
representation of a kelp bed. However in tall aquariums (1 meter
/ 36 inches), they would do very well given that a typical reef
aquarium and the keeping of fish and corals species that must be fed
provides for the kelp's requirement of both nitrogen and phosphorous,
that is if one is willing to take the risk of having this algae spread
to other aquariums within a reef system.
I also understand
that many hobbyists would find such a monospecific habitat to be
somewhat "boring" as the variety of fish and corals species would be
greatly reduced in comparison to a coral reef aquarium. For those
wishing to keep sea horses or pipefish, this habitat would suit them
extremely well as this is where they are most often found in nature.
Many mobile and sessile invertebrates would also do very well in
such a habitat, that is, as long as such animals do not have a need for
much light intensity as they will surely become shaded by the kelp.

As mentioned earlier, another concern if Sargassum is to be kept as
part of a reef aquarium's refugium is the spread of this algae into the
main coral display aquarium. I know of no fish species that we
typically keep in reef aquariums that would consume Sargassum kelp and
keep it under control. Other herbivores such as any of the
snails or sea urchins would most likely do little other than clearing
the Sargassum's blades of other algae species growing upon the kelp.
It is for these concerns that I can not recommend the keeping of a
tropical Sargassum refugium, but can however encourage the keeping of
such kelp as a very unique display of its own. I imagine such a
system stocked with sea horses and pipe fish would bring much enjoyment
to both you and any visitors with the surprise of realizing that a
seahorse is hiding in plain sight, while of course being the proper
habitat for such species.
Related Reading
:
A Philippine Fringing Reef &
The
Reef Aquarium Part One - Land meets Ocean
Part Two - The Grass is Always Greener.... Part Three - See The Weeds
An Online Philippine Reef Tour
The Reef Aquarium Clean Up Crew Acknowledgments :
I would like to thank my wife Linda for her loving
support and understanding of my interests in all things marine. A
special thank you goes out to Eric Borneman for his generosity in
providing assistance with this article and in helping me to make sense
of tropical reefs. To Dr. Ron Shimek and Leslie Harris, thank you for
the many identifications made as well as teaching me a great deal about
marine biology and zoology.
References:
Ang
P.O. (1986). Analysis of the vegetation structure of a Sargassum
community in the Philippines. Mar.Ecol.Prog.Series Vol. 28: 9-19
Atkinson
M.J. (1992). Effects of water velocity on phosphate uptake in
coral reef-flat communities. Limnol. Oceanogr., 37(2), 1992,
273-279
Choi
H.G. et al. (2007). Physiological differences in the growth of
Sargassum. J Appl Phycol. DOI 10.1007/s10811-007-9281-5
Deysher
L. , Norton T. (1982). Dispersal and colonization in Sargassum
muticum (Yendo) Fensholt. J. exp. mar. Biol.Ecol. 56: 179-195
Irving A.
D., Connell S. D. (2004).
Local complexity in patterns of canopy–benthos associations
produces regional patterns across temperate Australasia. Marine
Biology 144: 361–368
Kendrick
G.A. , Walker D.I. (1991). Dispersal distances for propagules of
Sargassum. Mar.Ecol.Prog.Series Vol.79:133-138, 1991
Schaffelke
B., Klumpp D.W. (1998) Nutrient-limited growth of the coral reef
macroalga Sargassum baccularia and experimental growth enhancement by
nutrient addition in continuous flow culture.
Mar.Ecol.Prog.Series Vol.164: 199-211
Schaffelke B. (2002) PARTICULATE ORGANIC MATTER AS AN ALTERNATIVE NUTRIENT SOURCE FOR TROPICAL SARGASSUM SPECIES. Journal of Phycology, Vol 35: 1150-1157
Stewart
H.L. (2006) Morphological variation and phenotypic plasticity of
buoyancy in the macroalga Turbinaria ornata across a barrier reef.
Marine Biology (2006) 149: 721–730 DOI
10.1007/s00227-005-0186-z.
Toohey,
B. et al. (2004) The effects of light and thallus scour from Ecklonia
radiata canopy on an associated foliose algal assemblage: the
importance of photoacclimation. Marine Biology (2004) 144:
1019–1027
Velimirov B. (1979) Wave-induced kelp movement and its importance for community structure. Bot Mar 22:169–172
© 2011 All Rights Reserved
Content
including photographs are copyright protected and may not be
used
or reproduced without written permission of the authors.










 The most commonly found fish species amongst the kelp,
the filefish with its camouflaged colorations and patterns along with a thin, vertical body
form allows these species to move through the kelp stands nearly
invisible to both predators and prey. I have noted two color
forms, a brown (as shown) and a mottled green, the previous found
exclusively amongst the kelp while the latter is commonly seen amongst
the macro algae and/or the zone between the macroalgae and kelp beds.
The most commonly found fish species amongst the kelp,
the filefish with its camouflaged colorations and patterns along with a thin, vertical body
form allows these species to move through the kelp stands nearly
invisible to both predators and prey. I have noted two color
forms, a brown (as shown) and a mottled green, the previous found
exclusively amongst the kelp while the latter is commonly seen amongst
the macro algae and/or the zone between the macroalgae and kelp beds. Seahorses are another common fish species found amongst the many
"hitching posts" that the kelp provides, that is, if one looks close
enough as they too are very well camouflaged to match their
surroundings. The seahorses relative, the pipefish, are also
found in large numbers as they too exploit the kelp structure for both
safety from predators and as a hunting ground.
Seahorses are another common fish species found amongst the many
"hitching posts" that the kelp provides, that is, if one looks close
enough as they too are very well camouflaged to match their
surroundings. The seahorses relative, the pipefish, are also
found in large numbers as they too exploit the kelp structure for both
safety from predators and as a hunting ground.  The Scribbled Rabbitfish is one of the few herbivores which are
found in any numbers within and near the Sargassum beds. I have
observed on many occasions, large schools of both juveniles and adults
grazing on the kelp's epiphytic growth, keeping the kelp blades free of
sun-blocking growths yet do not appear to consume any kelp. This
species is most sought after by local fishermen who place green algae
baited traps and remove large numbers of these fish each year.
Such removal of a herbivore is usually thought of as being
detrimental yet I believe that this fish species actually helps the
Sargassum by keeping its blades free of other growth while doing no
damage to the Sargassum itself.
The Scribbled Rabbitfish is one of the few herbivores which are
found in any numbers within and near the Sargassum beds. I have
observed on many occasions, large schools of both juveniles and adults
grazing on the kelp's epiphytic growth, keeping the kelp blades free of
sun-blocking growths yet do not appear to consume any kelp. This
species is most sought after by local fishermen who place green algae
baited traps and remove large numbers of these fish each year.
Such removal of a herbivore is usually thought of as being
detrimental yet I believe that this fish species actually helps the
Sargassum by keeping its blades free of other growth while doing no
damage to the Sargassum itself. 




 As mentioned earlier, another concern if Sargassum is to be kept as
part of a reef aquarium's refugium is the spread of this algae into the
main coral display aquarium. I know of no fish species that we
typically keep in reef aquariums that would consume Sargassum kelp and
keep it under control. Other herbivores such as any of the
snails or sea urchins would most likely do little other than clearing
the Sargassum's blades of other algae species growing upon the kelp.
As mentioned earlier, another concern if Sargassum is to be kept as
part of a reef aquarium's refugium is the spread of this algae into the
main coral display aquarium. I know of no fish species that we
typically keep in reef aquariums that would consume Sargassum kelp and
keep it under control. Other herbivores such as any of the
snails or sea urchins would most likely do little other than clearing
the Sargassum's blades of other algae species growing upon the kelp. 