
Presented by Charles & Linda Raabe
http://www.chucksaddiction.com/
Mactan Island, The Philippines
© 2009 All Rights Reserved
Introduction:
The infraorder Caridea is the largest shrimp group in the world, containing almost 2,800 described species with estimates of twice that number of species yet to be discovered and described. Although they occur in all of the world's oceans, the Caridean shrimp reach their highest level of species and generic diversification in the Indo-Pacific. Within this region, they are only rivaled in their species diversity by the Alpheidae, earning them a place within this online resource as well.
In the tropical marine environment many small species have evolved specialized lifestyles, living commensally on and within various species of sponges, corals, echinoderms and polychaetes, while others lead a more free-ranging lifestyle. These various lifestyles are what I find the most interesting and hope to continue my study of these relationships.
Coloration :
Since it is my goal to examine and present the live colorations of the shrimp that I am able to collect in addition to their morphology, it is important to try and understand why the various species develop coloration or not and that there can be great variation of colors and patterns within a species.
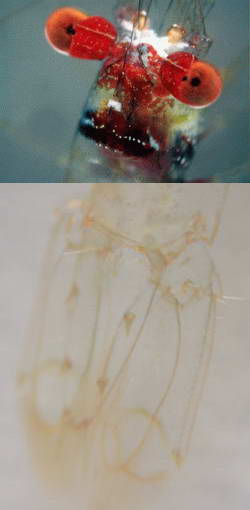 The exoskeleton of many carideans are completely
transparent, especially with juveniles or very small species yet there
are species that are very colorful as shown in the example.
These colors are derived from pigments that are either located
within the exoskeleton or within cells below the exoskeleton. Without
such pigmentation the carideans would appear transparent except for the
black of their eyes, opaque internal organs and food filled stomachs
and digestive tracts, of which can give the shrimp a green coloration
due to the visible green algae that they have consumed.
The exoskeleton of many carideans are completely
transparent, especially with juveniles or very small species yet there
are species that are very colorful as shown in the example.
These colors are derived from pigments that are either located
within the exoskeleton or within cells below the exoskeleton. Without
such pigmentation the carideans would appear transparent except for the
black of their eyes, opaque internal organs and food filled stomachs
and digestive tracts, of which can give the shrimp a green coloration
due to the visible green algae that they have consumed. In areas of the shrimp that are transparent, such as the mouthparts, appendages and tail fans, they may appear a light amber coloration. These golden hues are due to a hardening of the exoskeleton's outer layers known as sclerotization, a chemical process also known as quinone tanning. This hardening of the outer layers protects the
exoskeleton from wear and tear while giving the shrimp a bit of color, although this type of coloration can not be used for identification purposes as all shrimp are subject to sclerotization in one degree or another.
Live coloration becomes important as the preserved specimens of many closely related species are so similar in their morphological traits that species cannot be distinguished. Color patterns exhibited in life often clearly show species differences that the morphology of preserved specimens cannot.
A very important caveat in coloration and patterns of coloration is that variations of those colors do not always mean that there are distinct, separate species (Bauer 2004). Color variations can also be environmentally determined by background. A good case in point is Periclimenes commensalis, a commensal of Crinoids that themselves have varied colorations which the shrimp are able to mimic exactly, meaning that a single species can appear in any number of colors yet tend to follow the same species patterns of coloration. On a single crinoid I found two P. commensalis showing this ability, both having a black background coloration yet showed a variation in the yellow coloration of their pattern. One being an orange pattern and the other a yellow.
Obviously if color morphs alone were recognized as individual species then the understanding of their ecology, behavior and evolution would be very confused. But if color patterns vary among morphologically similar individuals living in the same geographic area and have consistent differences in other traits such as behavior, preferred habitat and mate choice, then that could be an indication of different species. Of course such close similaritys would always cast doubt upon their actually being different species yet with todays ability to test DNA differences, such doubts can now be much more easily confirmed or ruled out regarding a species status.
The superfamily Palaemonoidea are of greater interest to me as they make themselves easily visible or at the least, easily found, which is evident by the species I have collected and photographed below. This single superfamily has enough members that one could spend a life time learning of their many morphological differences, meaning that I will most likely be quite busy and intrigued for many years to come. I expect in due time to add many more family groups within this superfamily as collecting luck will have it.
It is our goal to provide the best possible photographic identification features with emphasis on live colorations while providing enough taxonomy details to be of an aide in identifying other specimens. Photographic quality is of course fully dependent on not only my available equipment, but my skills with said equipment and my ability to obtain specific details through dissection, which should improve as I gain experience and of course, patience.
PLEASE NOTE : When I have but a single specimen to work with the amount of and quality of photographic details will most likely suffer as I intend to keep single specimens intact as much as possible in case the specimen is needed for examination by others much more qualified to do so.
Acknowledgments : A special Thank you goes out to :
Dr. A.J. Bruce of the Queensland Museum, Australia
Dr. Ivan N. Marin of the A.N. Severtzov Institute of Ecology and Evolution, Moscow, Russia
Dr. A. Anker and Leslie Harris of the Los Angeles Natural History Museum for their kind assistance with identifications. Their time and efforts are greatly appreciated.
Classification :
Kingdom : Animalia Phylum : Arthropoda Subphylum : Crustacea Class : Malacostraca Order : Decapoda Suborder : Pleocyemata Infraorder : Caridea
Superfamilies : Alpheoidea - Atyoidea - Bresilioidea - Campylonotoidea - Caridea Incertae Sedis - Crangonoidea - Nematocarcinoidea - Oplophoroidea - Palaemonoidea - Pandaloidea - Pasiphaeoidea - Procaridoidea - Processoidea - Stylodactyloidea
Super Family - Alpheoidea Rafinesque, 1815
Family Groups - Alpheidae - Barbouriidae - Hippolytidae - Ogyrididae
Family - Alpheidae Rafinesque, 1815
Genus Groups - Alpheopsis - Alpheus - Amphibetaeus - Arete - Aretopsis - Athanas - Athanopsis - Automate - Betaeopsis - Betaeus - Deioneus - Fenneralpheus - Leptalpheus - Metalpheus - Neoalpheopsis - Parabetaeus - Potamalpheops - Racilius - Salmoneus - Salomeus - Synalpheus - Thunor
Reference(s):
Genus - Alpheus Fabricius, 1798
Species - A. aculeipes - A. acutocarinatus - A. acutofemoratus - A. affinis - A. albatrossae - A. albatrossi - A. alcyone - A. alcyone de - A. amblyonix - A. amblyonyx - A. amirantei - A. amirantei sizou - A. angulosus - A. angustodigitus - A. architectus - A. arethusa - A. armatus - A. armillatus - A. astrinx - A. audouini - A. australiensis - A. australosulcatus - A. avarus - For the complete listing, click HERE.


A. lottini (Alph001) A. cf frontalis (CAR002)



Alpheus spp. #1 (CAR010) Alpheus leviusculus (CAR020) Alpheus spp. #2 (CAR029)
Genus - Athanas
Species - A. nitescens

Athanas spp. (CAR025)
Genus - Synalpheus Bate, 1888
Species - S. acanthitelsonis - S. agelas - S. albatrossi - S. anasimus - S. ancistrorhynchus - S. apioceros - S. bannerorum - S. bispinosus - S. bituberculatus - S. biunguiculatus - S. bousfieldi - S. brevicarpus - S. brevifrons - S. brooksi - S. carinatus - S. charon - S. comatularum - S. coutierei - S. coutierei, - S. crosnieri - S. demani - S. digueti - S. disparodigitus - S. dominicensis - S. dorae - S. echinus - S. filidigitus - S. fossor - S. fritzmuelleri - S. gambarelloides - S. gamberelloides - S. goodei - S. gracilirostris - S. grampusi - S. gravieri - S. harpagatrus - S. hastilicrassus - S. heardi - S. hemphilli - S. heroni - S. herricki - S. iocasta - S. laevimanus - S. laticeps - S. lockingtoni - S. longicarpus - S. macclendoni - S. macromanus - S. minus - S. modestus - S. mulegensis - S. neptunus - S. neptunus germanus - S. neptunus neptunus - S. nobilii - S. occidentalis - S. odontophorus - S. pachymeris - S. pandionis - S. paraneomeris - S. paraneptunus - S. pectiniger - S. pescadorensis - S. pococki - S. quadriarticulatus - S. quinquedens - S. rathbunae - S. readi - S. redatocarpus - S. sanctithomae - S. scaphoceris - S. sciro - S. spinifrons - S. stimpsoni - S. stimpsonii - S. streptodactylus - S. tanneri - S. thai - S. theano - S. tijou - S. townsendi - S. tropidodactylus - S. tumidomanus


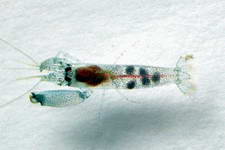
S. stimpsoni (Syna001) Synalpheus spp. #1 (CAR018) Synalpheus spp. #2 (CAR019)
Family - Hippolytidae Dana, 1852
Genus Groups - Bythocaris - Calliasmata - Caridion - Chorismus - Eualus - Exhippolysmata - Heptacarpus - Hetairus - Hippolysmata - Hippolytidae - Hippolyte - Latreutes - Lebbeus - Leptodius - Lysmata - Merhippolyte - Parhippolyte - Saron - Spirontocaris - Thinora - Thor - Thoralus - Tozeuma - Trachycaris
Reference(s):
Genus - Hippolyte Leach, 1814
Species - H. australiensis - H. acuta - H. bifidirostris - H. californiensis - H. caradina - H. clarki - H. coerulescens - H. commensalis - H. edmondsoni - H. ignobilis - H. inermis - H. nicholsoni - H. obliquimanus - H. pleuracanthus - H. prideauxiana - H. varians - H. ventricosa - H. zostericola



Hippolyte #1 (CAR 033) Hippolyte #2 (CAR 039) Hippolyte cf. commensalis (CAR 032)
Genus - Latreutes Stimpson, 1860
Species - L. acicularis - L. anoplonyx - L. compressus - L. ensifer - L. ensiferus - L. fucorum - L. inermis - L. laminirostris - L. mucronatus - L. parvulus - L. planirostris - L. porcinus - L. pygmaeus - L. pymoeus

Latreutes spp. (Latr001)
Genus - Saron Thallwitz, 1888
Species - S. marmonratus - S. neglectus

Saron specimen 1 (CAR008)
Genus - Thor De Man, 1888
Species - T. amboinensis - T. dobkini - T. floridanus - T. manningqi - T. paschalis - T. spinosus

T. amboinensis (CAR 012)
Super Family - Palaemonoidea Rafinesque, 1815
Family Groups - Anchistioididae - Desmocarididae - Euryrhynchidae - Gnathophyllidae - Hymenoceridae - Kakaducarididae - Palaemonidae - Typhlocarididae
Family - Gnathophyllidae Guérin-Méneville, F.É. 1856
Genus Groups - Gnathophylloides - Gnathophyllum - Hymenocera - Levicaris

G. americanum (CAR044)
Family - Palaemonidae Rafinesque, 1815
Genus groups - Calathaemon - Climeniperaeus - Cryphiops
SubFamilies - Palaemoninae Rafinesque, 1815
Genus Groups - Brachycarpus - Exopalaemon - Leander - Leandrites - Leptocarpus - Macrobrachium - Nematopalaemon - Palaemon - Palaemonetes - Urocaridella
- Pontoniinae Kingsley, 1878
Genus Groups - Allopontia - Altopontonia - Amphipontonia - Anapontonia - Anchistus - Apopontonia - Araiopontonia - Balssia - Carinopontonia - Chacella - Chernocaris - Conchodytes - Coralliocaris - Coutierea - Crinotonia - Ctenopontonia - Cuapetes - Dasella - Dasycaris - Diapontonia - Epipontonia - Eupontonia - Exopontonia - Fennera - Hamiger - Hamodactyloides - Hamodactylus - Hamopontonia - Harpiliopsis - Ischnopontonia - Isopontonia - Jocaste - Laomenes - Lipkebe - Manipontonia - Mesopotonia - Metapotonia - Miopotonia - Neoanchistus - Neopontonides - Notopontonia - Onycocaridella - Onycocaridites - Onycocaris - Orthopontonia - Palaemonella - Paraclimenaeus - Paranchistus - Parapontonia - Paratypton - Periclimenaeus - Periclimenella - Periclimenes - Pericliminoides - Philarius - Platycaris - Platypontonia - Plesiopontonia - Pliopontonia - Pontonia - Pontonides - Pontoniopsis - Propontonia - Pseudocoutierea - Pseudopontonides - Rapipontonia - Sandimenes - Stegopontonia - Tectopontonia - Thaumastocaris - Tuleariocaris - Typton - Veleronia - Veleroniopsis - Vir - Waldola - Yeminicaris - Zenopontonia
Genus - Conchodytes Peters, 1852
Species - C. kempi - C. maculatus - C. meleagrinae - C. monodactylus - C. nipponensis - C. tridacnae

C. meleagrinae ( CAR 016 )
(tentative identificaton)
Genus - Coralliocaris Stimpson, 1860
Species - C. brevirostris - C. graminea - C. macropthalma - C. nudirostris - C. pavonae - C. superba - C. taiwanensis - C. venusta - C. viridis
Genus - Crinotonia Marin, 2006*
* "Differs from other pontoniine genera in the combination of several morphological features, including the absence of a podobranch on the second maxilliped, an arthrobranch on the third maxilliped; the presence of several robust disto-lateral and ventro-mesial teeth on the first segment of the antennular peduncle; the extreme dimorphism of the second pereiopods; third pereiopod with propodus having a serrated ventral margin, without distoventral spines, with a specialized dactylus. Crinotonia is similar to the genus Pontoniopsis which is also associated with Crinoid hosts."
Species - C. anastasiae - C. attenuatus
*Reference : Dr. Ivan Marin (2006), Description of Crinotonia anastasiae, New genus, New species, A new crinoid associated Pontoniine shrimp from Nha Trang Bay, Vietnam. The Raffles Bulletin of Zoology 2006 54(2): 321-340.

C. attenuatus (Croin001)
Genus - Cuapetes Okuno, 2009 *
Species: Currently, this genus consists of the following 24 species all previously assigned to Kemponia (see Bruce 2004; Li 2008) in addition to the type species, Cuapetes nilandensis (Borradaile, 1915) - Cuapetes agag (Kemp, 1922) - Cuapetes akiensis (Kubo, 1936) - Cuapetes americanus (Kingsley, 1878) - Cuapetes amymone (De Man, 1902) - Cuapetes anacanthus (Bruce, 1989) - Cuapetes andamanensis (Kemp, 1922) - Cuapetes calmani (Tattersall, 1921) - Cuapetes darwiniensis (Bruce, 1987) - Cuapetes demani (Kemp, 1915) - Cuapetes edwardsi (Paulson, 1875) - Cuapetes elegans (Paulson, 1875) - Cuapetes ensifrons (Dana, 1852) - Cuapetes grandis (Stimpson, 1860) - Cuapetes johnsoni (Bruce, 1987) - Cuapetes kororensis (Bruce, 1977) - Cuapetes lacertae (Bruce, 1992) - Cuapetes longirostris (Borradaile, 1915) - Cuapetes paulsoni (Bruce, 2003) - Cuapetes platycheles (Holthuis, 1952) - Cuapetes rapanui (Fransen, 1987) - Cuapetes seychellensis (Borradaile, 1915) - Cuapetes suvadivensis (Borradaile, 1915) - Cuapetes tenuipes (Borradaile, 1898) - Cuapetes ungujaensis (Bruce, 1969).
*Reference : Junji Okuno - Cuapetes Clark, 1919, a senior synonym of Kemponia Bruce, 2004. Zootaxa 2028: 67–68 2009







C. cf. seychellensis (Kemp006) Cuapetes species 1 (CAR001) C. cf. grandis (CAR005)
The C. tunuipes complex, an unresolved identity case.



C. cf tenuipes (Kemp002) C. cf tenuipes ( CAR 003 ) C. cf tenuipes (CAR 038)
Genus - Exoclimenella Bruce, 1994
Species - E. denticulatus - E. maldivensis - E. sibogae

E. maldivensis (exo002)
Genus - Hamodactylus, Holthuis 1952
Species - H. aqabai - H. boschmai - H. noumeae

H. noumeae (hamod001)
Genus - Hamopontonia Bruce, 1970
Species - H. corallicola - H. essingtoni

Hamopontonia ?sp. (Hamo001)
Genus - Harpiliopsis Borradaile, 1917
Species - H. beaupresii - H. depressa - H. spinigera


H. beaupresii (Harp001) H. depressa (Harp002)
Genus - Harpilius Dana, 1852
* "The genus Harpilius appears particularly closely related to two other pontoniine genera that are also obligatory scleractinian associates, Vir and Philarius. Their general morphologies are very similar, and all have a well developed finger-like medial process on the fourth thoratic sternite. All species of these genera have simple hamate ambulatory dactyls."
Species: H. bayeri - H. consobrinus - H. lutescens
* Reference: A.J. Bruce (2004). A partial revision of the genus Periclimenes Costa, 1884. Zootaxa 582: 1-26.

H. consobrinus (Harpil 001)
Genus - Jocaste Holthuis, 1952
Species - J. japonica - J. lucina - J. platysoma
Genus - Palaemonella Dana, 1852
Species - P. atlantica - P. crosnieri - P. elegans - P. lata - P. maziwi - P. pottsi - P. pusillus - P. rotumana - P. spinulata - P. tenuipes



P. pottsi (Palae001) P. aff. pottsi (NS002) Palaemonella spp. (CAR 031)
Genus - Periclimenaeus Borradaile, 1915
Species - P. arabicus - P. ardeae - P. arthrodactylus - P. ascidiarum - P. atlanticus - P. bermudensis - P. bidentatus - P. bouvieri - P. bredini - P. caraibicus - P. chacei - P. crassipes - P. diplosomatis - P. djiboutensis - P. garthi - P. gorgonidarum - P. hancocki - P. hebedactylus - P. hecate - P. holthuisi - P. jeancharcoti - P. leptodactylus - P. lobiferus - P. manihinei - P. maxillulidens - P. minutus - P. nobilii - P. orbitospinatus - P. orontes - P. pachydentatus - P. pacificus - P. palauensis - P. pearsei - P. perlatus - P. quadridentatus - P. rastrifer - P. rhodope - P. robustus - P. schmitti - P. spinicauda - P. spinimanus - P. spinosus - P. spongicola - P. storchi - P. stylirostris - P. tchesunovi - P. tridentatus - P. trispinosus - P. truncatus - P. tuamotae - P. uropodialis - P. usitatuswilsoni - P. zanzibaricus - P. zarenkovi

Periclimenaeus storchi ( CAR 013)
Genus - Periclimenella Bruce, 1994
Species - P. petitthouarsii - P. spinifera

P. spinifera (Peric001)
Genus - Periclimenes Costa, 1844
Species: P.adularans - P.aegylios - P.aesopius - P.affinis - P.albatrossae - P.alcocki - P.aleator - P.amethysteus - P.andamanenesis - P. andresi - P.anthophilus - P.antonbruuni - P.batei - P.bowmani - P.brevicarpalis - P.brevicarpalus - P.brevinaris - P.brevirostris - P.brocketti - P.brockii - P.brucei - P.calcaratus - P.calcartus - P.carinidactylus - P.colemani - For the complete listing, Click HERE.
Reference:




P. brevicarpalis ( CAR 007 ) P. commensalis ( CAR 011 ) P. grandidens ( CAR 022 ) P. ornatus ( CAR 041 )



Genus - Pontonides Borradaile, 1917
Species - P. ankeri - P. sympathes - P. unciqer

P. ankeri ( CAR 014 )
Genus - Rapipontonia Marin, 2007
Species - R. galene - R. paragalene

R. galene (Rapi001)
Genus - Sandimenes Li, 2009
Species - S. hirsutus

S. hirsutus (CAR 042)
Genus - Thaumastocaris Kemp, 1922
Species - T. streptopus

T. streptopus (CAR 040)
Genus - Vir Holthuis, 1952
* " The genus Vir was erected for Palaemonella orientalis. Since then four species have been described and existence of further undescribed species has been mentioned. The two most recently described species, V. euphyllius and V. pareuphyllius were found in Nha Trang Bay, southern Vietnam, in association with hammer corals of the genus Euphyllia. These two species appeared to be morphologically very similar, differing mainly in the shape of the rostrum, different configuration of lobes on the distal margin of the carpus of the second pereiopods, and the size of the mandibular palp."
Species : V. philippinensis - V. colemani - V. euphyllia - V. pareuphyllius
* Reference material - Vir euphyllia.pdf


Super Family - Pandaloidea Haworth, 1825
Family Group - Pandalidae Haworth, 1825
Genus Groups - Atlantopandalus - Chlorotocus - Dichelopandalus - Heterocarpoides - Heterocarpus - Pandalidae - Pandalina - Pandalopsis - Pandalus - Pantomus - Parapandalus - Plesionika - Stylopandalus
Reference(s):
Genus - Chlorotocus Costa, 1871
Species - C. jactans


C. jactans (Chlor001) 2nd specimen (CAR 037)
Infraorder - Stenopodidea Claus, 1872
Family - Stenopodidae Claus, 1872 Genus Groups - Engystenopus - Microprosthema - Odontozona - Richardina - Spongicoloides - Stenopus
Genus - Microprosthema Stimpson, 1860
Species - M. emmiltum - M. granatense - M. jareckii - M. plumicorne - M. semilaeve

M. plumicorne (micro001)
Symbiosis (Greek words meaning "living together") Is applied to any association which there is prolonged close contact between individuals of two species. The symbiont is the species that has abandoned its free-living habits to live with another, usually larger species that is termed the host. Different forms of symbiosis are defined by the costs and benefits (if any) to both species as explained in the following terms.
Amensalism = two organisms living together with no specific benefit to either. The relationship is neutral. Generally, something like a duck living next to a cat-tail or other wet land plant would fit here. The two organisms live together due to random events; any specific benefits to either party are so small as to be inconsequential.
Commensalism = two organisms living together, with one benefiting, the other is neutral.
Mutualism = two organisms living together, with both benefiting for the mutual good. Zooxanthellae (algae) in corals is a good example.
Parasitism = two organisms living together, one benefits, the other suffers, but it is non-lethal.
Coral Symbionts
 In the Indo-Pacific, the scleractinian (reef-building) corals appear to
be very important hosts for symbiotic shrimps, providing cover in
which the shrimp can hide along with the coral's stinging tentacles
providing protection from predators and therefore are at the least,
commensal. However, many shrimp symbionts have given up the
free-ranging life style and remain at all times within the protection
of the coral and feed either directly on the coral's mucus or take food
particles that the coral has captured making the shrimp a parasite of
sorts, although the coral most likely suffers very little due to the
shrimp's feeding habits and it may be a good trade off for the coral as
the shrimp also performs some service to the coral in the way of
clearing sediments and possibly warding off coral parasites such as
some nudibranch and copepod species.
In the Indo-Pacific, the scleractinian (reef-building) corals appear to
be very important hosts for symbiotic shrimps, providing cover in
which the shrimp can hide along with the coral's stinging tentacles
providing protection from predators and therefore are at the least,
commensal. However, many shrimp symbionts have given up the
free-ranging life style and remain at all times within the protection
of the coral and feed either directly on the coral's mucus or take food
particles that the coral has captured making the shrimp a parasite of
sorts, although the coral most likely suffers very little due to the
shrimp's feeding habits and it may be a good trade off for the coral as
the shrimp also performs some service to the coral in the way of
clearing sediments and possibly warding off coral parasites such as
some nudibranch and copepod species. Echinoderm Symbionts
 Quite
a number of shrimp species take advantage of Echinoderm's defensive
protection and gain not only the same protection from predators as the
Echinoderm does, but also benefits from being on or within a mobile
invertebrate that allows the shrimp to gather food as it either moves
along the substrates or the shrimp simply steals food particles that
the Echinoderm has collected.
Quite
a number of shrimp species take advantage of Echinoderm's defensive
protection and gain not only the same protection from predators as the
Echinoderm does, but also benefits from being on or within a mobile
invertebrate that allows the shrimp to gather food as it either moves
along the substrates or the shrimp simply steals food particles that
the Echinoderm has collected. Food for symbiotic shrimp may be as important as shelter in explaining their association with Echinoderms. Not only do the shrimp take advantage of passing or collected food particles they also have a ready food supply in the way of superficial tissues and associated mucus covering calcareious plates and spines of the Echinoderm host.
Species of the family Gnathophyllidae are often associated with Echinoderms although are considered free living but are often found near or on sea urchins or other Echinoderms. Those Gnathophyllum species not permanently associated with their possible hosts are most likely micropredators rather than symbionts tending towards parasitism.
Anemone Symbionts
 As
with other shrimp genera utilizing various hosts as described above,
there are a number of species that use anemone as their home.
Some live at the base of the anemone while others are a bit more
bold and stay within the stinging tentacles of the anemone, gaining
protection from fish predators as most fish will not brave being stung
by the anemone. As with other relationships, these shrimp may
also benefit by feeding on the anemone's mucus and regurgitated
undigested food. If the shrimp receives protection and food from
the anemone, it is a commensal, but most likely leans towards
parasitism.
As
with other shrimp genera utilizing various hosts as described above,
there are a number of species that use anemone as their home.
Some live at the base of the anemone while others are a bit more
bold and stay within the stinging tentacles of the anemone, gaining
protection from fish predators as most fish will not brave being stung
by the anemone. As with other relationships, these shrimp may
also benefit by feeding on the anemone's mucus and regurgitated
undigested food. If the shrimp receives protection and food from
the anemone, it is a commensal, but most likely leans towards
parasitism. It is doubtfull that the anemone recieves significant benefit from the shrimp although the nitrogenous excretions of the shrimp can slightly increase the abundance of zooxanthellae in the tissue of its anemone host. If the shrimp's fertilization of the zooxanthellae significantly boosts the zooxanthellae's energy contribution to the host, then the symbiosis between the shrimp and the anemone is mutualistic.
Sponge Symbionts
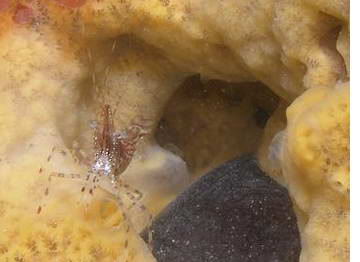 Sponges have a variety of canals and spaces used to carry
oxygenated water and suspended particles that serve as food for the
sponge. They also produce some very noxious compounds that repel
predators and thus make for a safe and convienent home for a number of
shrimp species. However, little is known about the true
relationship of these species to their sponge hosts. Studies by
Duffy have however revealed quite a bit about the basic biology and
evolution of sponge associates of the genus Synalpheus, several of
which are obligate associates of sponges with some of them feeding
directly on the sponge's tissue, making the relationship more parasitic
than commensal. Duffy has also shown how the combination of
symbiotic living on different hosts and abbreviated-direct development
has led to much speciation in this genus. Eusocialty, which is
known in only a few animal groups has evolved in some Synalpheus
symbionts of sponges.
Sponges have a variety of canals and spaces used to carry
oxygenated water and suspended particles that serve as food for the
sponge. They also produce some very noxious compounds that repel
predators and thus make for a safe and convienent home for a number of
shrimp species. However, little is known about the true
relationship of these species to their sponge hosts. Studies by
Duffy have however revealed quite a bit about the basic biology and
evolution of sponge associates of the genus Synalpheus, several of
which are obligate associates of sponges with some of them feeding
directly on the sponge's tissue, making the relationship more parasitic
than commensal. Duffy has also shown how the combination of
symbiotic living on different hosts and abbreviated-direct development
has led to much speciation in this genus. Eusocialty, which is
known in only a few animal groups has evolved in some Synalpheus
symbionts of sponges. Molluscan-Living Shrimps
 Mantle cavities of molluscs, especially the bivalves such as
clams and oysters make for a good habitat for symbiotic shrimp.
The cavities, which houses the gills of the mullusc, not only
function in respiration but also in food collection of filter-feeding
bivalves.
Mantle cavities of molluscs, especially the bivalves such as
clams and oysters make for a good habitat for symbiotic shrimp.
The cavities, which houses the gills of the mullusc, not only
function in respiration but also in food collection of filter-feeding
bivalves. While providing obvious protection to the shrimp, the shrimp are also provided food in the form of mucus and/or food particles filtered out of the water by the gills of the bivalve. Species of the Pontoniine genera Conchodytes, Anchistus, Paranchistus and Platypontonia along with others, occupy this niche.
The above relationships are but a small sampling as there are a great many more such relationships. I have come to learn that regardless of where I look, there is bound to be a shrimp taking advantage of both shelter and food sources. Be it within clams, tunicates and sponges to living on the surfaces of gorgonians and anemone along with many free-ranging species it is of no surprise that if one stops and looks closely, anywhere, the odds are in favor of you finding a shrimp.
References:
Bauer, Raymond T. Remarkable shrimps : adaptations and natural history of the Carideans. University of Oklahoma Press, Norman, Publishing 2004
Bruce, A.J. 1975. Coral reef shrimps and their colour patterns. Endeavour 34: 23-27
Chace, F.A. Bruce A.J. The Caridean Shrimps (Crustacea: Decapoda) of the Albatross Philippine Expedition, 1907-1910. Smithsonian Institution Press - 1997 - si-pddr.si.edu



