

 As with any marine species, water quality plays
an important role
in ensuring they remain healthy. Being an invertebrate, shrimp do not
tolerate anything less than reef grade water. If you can provide
consistent
water quality, you should have no trouble at all in being able to keep
your pet healthy and free of problems.
As with any marine species, water quality plays
an important role
in ensuring they remain healthy. Being an invertebrate, shrimp do not
tolerate anything less than reef grade water. If you can provide
consistent
water quality, you should have no trouble at all in being able to keep
your pet healthy and free of problems.

 This may not be easy for those that do not have access to
a variety of starfish. While I have the luxury of being able to collect
any starfish myself. For those of you that have to purchase starfish in
order to feed these shrimp, you may wish to use some of the more
prolific starfish such as the common Asterina species found commonly
hitch hiking in on purchased live rock. Over the course of time, I have
collected a variety of starfish species in order to determine which are
found palatable or not, to the shrimp.
This may not be easy for those that do not have access to
a variety of starfish. While I have the luxury of being able to collect
any starfish myself. For those of you that have to purchase starfish in
order to feed these shrimp, you may wish to use some of the more
prolific starfish such as the common Asterina species found commonly
hitch hiking in on purchased live rock. Over the course of time, I have
collected a variety of starfish species in order to determine which are
found palatable or not, to the shrimp.





 Depending upon their health and quality of diet, the harlequin
shrimp will molt an average of once each month. Prior to molting you
can see the exoskeleton looking like an old dirty "skin". This seems to
cause the shrimp great irritation just prior to molting. They will
constantly pick at themselves as if trying to speed up the process by
making the exoskeleton more loose by their tugging at it.
Depending upon their health and quality of diet, the harlequin
shrimp will molt an average of once each month. Prior to molting you
can see the exoskeleton looking like an old dirty "skin". This seems to
cause the shrimp great irritation just prior to molting. They will
constantly pick at themselves as if trying to speed up the process by
making the exoskeleton more loose by their tugging at it.

 This only occurs directly after the female has broadcast her spawn.
Once all of the larvae have hatched, it is normal for her to molt the
next day. Once she has molted she is immediately ready to form a new
clutch of eggs, but first needs the male's contribution as the eggs are
fertilized as they pass by the deposited spermatophores. The male will
lift the female's tail and turn himself onto his back and join with the
female. It is at this time that the male applies spermatophores or
sperm sacs close to the opening of the female's genital duct. The sperm
sacs are shed from a pair of holes at the base of the last legs and the
eggs from holes on the third legs.
This only occurs directly after the female has broadcast her spawn.
Once all of the larvae have hatched, it is normal for her to molt the
next day. Once she has molted she is immediately ready to form a new
clutch of eggs, but first needs the male's contribution as the eggs are
fertilized as they pass by the deposited spermatophores. The male will
lift the female's tail and turn himself onto his back and join with the
female. It is at this time that the male applies spermatophores or
sperm sacs close to the opening of the female's genital duct. The sperm
sacs are shed from a pair of holes at the base of the last legs and the
eggs from holes on the third legs.


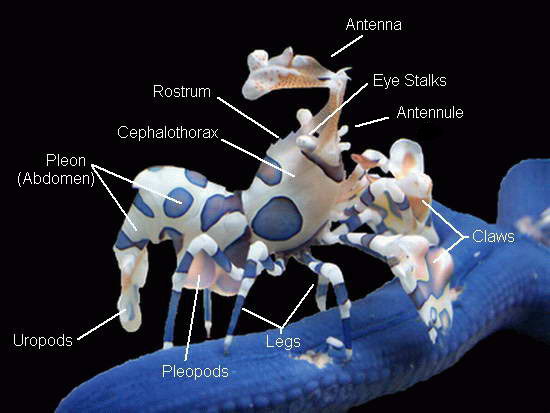
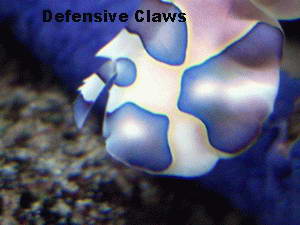
 When compared to other shrimp species, the harlequin shrimp seems to
come with some extra parts, or parts that are not easily identified as
to what they are due to their flamboyant structuring. Trying to find,
let alone determine, what the various head and claw structures are can
be a bit of a challenge as the shrimp tends to shield itself, making
its smaller details difficult to see.
When compared to other shrimp species, the harlequin shrimp seems to
come with some extra parts, or parts that are not easily identified as
to what they are due to their flamboyant structuring. Trying to find,
let alone determine, what the various head and claw structures are can
be a bit of a challenge as the shrimp tends to shield itself, making
its smaller details difficult to see.
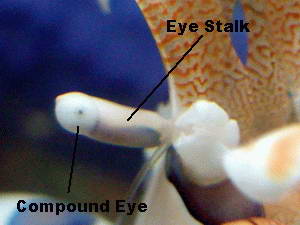 The
eyes appear to be well developed and allow the shrimp to make out
details, if the object is close enough. From a distance, they most
likely can only make out movement and the differences between light and
dark objects.
The
eyes appear to be well developed and allow the shrimp to make out
details, if the object is close enough. From a distance, they most
likely can only make out movement and the differences between light and
dark objects. 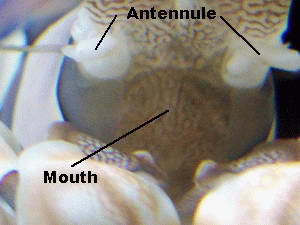 The mouth is not readily visible since their food particles
are
extremely small, hence no need for a large opening. The mouth can be
seen as the vertical slit shown in the photo.
The mouth is not readily visible since their food particles
are
extremely small, hence no need for a large opening. The mouth can be
seen as the vertical slit shown in the photo.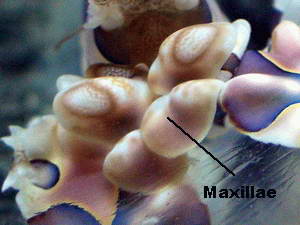 The
Maxillipeds are appendages modified to function as mouth parts in some
shrimp species. I have not seen the Harlequin shrimp use them as such.
In this species they may serve more as a means to recognize friend from
foe as mated pairs often touch with their maxillae as if taste testing
each other.
The
Maxillipeds are appendages modified to function as mouth parts in some
shrimp species. I have not seen the Harlequin shrimp use them as such.
In this species they may serve more as a means to recognize friend from
foe as mated pairs often touch with their maxillae as if taste testing
each other.







 Since my wife and I live in a small apartment, I found it
necessary to limit the size and scope of my breeding program by
designing a simple, compartmented kreisel design. Such a design allows
me to keep the station near my reef aquarium. As I stated earlier, I
use water from my reef for partial water changes to the breeding
station. Of course, you can use prepared salt mixes when doing the
daily partial water changes, but I prefer to use the water from my reef
aquarium as it makes the chore a very simple matter. If you do the
same, please ensure that your reef aquarium's water is of very high
quality. Any problems with phosphate and nitrate levels will have a
very detrimental effect on the larvae.
Since my wife and I live in a small apartment, I found it
necessary to limit the size and scope of my breeding program by
designing a simple, compartmented kreisel design. Such a design allows
me to keep the station near my reef aquarium. As I stated earlier, I
use water from my reef for partial water changes to the breeding
station. Of course, you can use prepared salt mixes when doing the
daily partial water changes, but I prefer to use the water from my reef
aquarium as it makes the chore a very simple matter. If you do the
same, please ensure that your reef aquarium's water is of very high
quality. Any problems with phosphate and nitrate levels will have a
very detrimental effect on the larvae.




 This is normally quite easy since in the wild mated pairs are commonly
collected and kept together to be shipped out and sold as mated pairs.
Obtaining such a pair should be a simple matter of doing an online
search or having your local store order a pair for you. If you only
need a single individual, should one of the pair die, then the store
should be able to distinguish between the sexes, allowing you to order
a male or female replacement. If the store employees do not know how to
sex the harlequin shrimp, you can describe how to do so (described
below) or you can shop elsewhere if you are not comfortable with their
ability to do so.
This is normally quite easy since in the wild mated pairs are commonly
collected and kept together to be shipped out and sold as mated pairs.
Obtaining such a pair should be a simple matter of doing an online
search or having your local store order a pair for you. If you only
need a single individual, should one of the pair die, then the store
should be able to distinguish between the sexes, allowing you to order
a male or female replacement. If the store employees do not know how to
sex the harlequin shrimp, you can describe how to do so (described
below) or you can shop elsewhere if you are not comfortable with their
ability to do so. Once
you know what to look for it is fairly easy to tell the sexes apart.
When mature, the female will appear obviously larger than the male. The
best, and most accurate method is by getting a look under their
abdomens. The male's abdomen will be clear or yellowish in color,
lacking any blue spots. The female will have obvious blue spots under
her abdomen and usually the very telling egg mass as well.
Once
you know what to look for it is fairly easy to tell the sexes apart.
When mature, the female will appear obviously larger than the male. The
best, and most accurate method is by getting a look under their
abdomens. The male's abdomen will be clear or yellowish in color,
lacking any blue spots. The female will have obvious blue spots under
her abdomen and usually the very telling egg mass as well.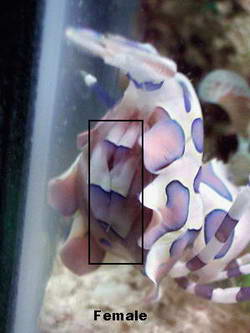


 While in the process of laying a new clutch of eggs, the female may go
into hiding for a day or two. I have never seen the actual egg laying
take place, yet am always happy to see her return with a new clutch of
eggs. While the female is carrying eggs, she will paddle her pleopods
quite often in order to aerate the eggs. She will also spend a good
deal of time picking at the eggs, which I assume is done to keep them
clean of any debris and to remove any unfertilized eggs.
While in the process of laying a new clutch of eggs, the female may go
into hiding for a day or two. I have never seen the actual egg laying
take place, yet am always happy to see her return with a new clutch of
eggs. While the female is carrying eggs, she will paddle her pleopods
quite often in order to aerate the eggs. She will also spend a good
deal of time picking at the eggs, which I assume is done to keep them
clean of any debris and to remove any unfertilized eggs.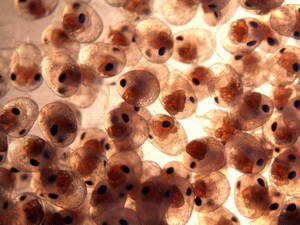
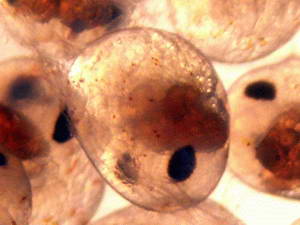

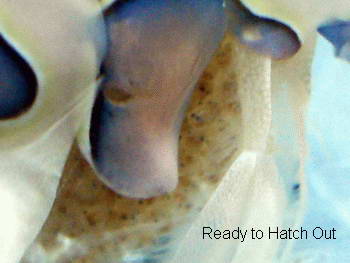
 When the female senses that the time is near, she will climb to the
highest point of the rock work during the middle of the night, usually
between 1:00 – 3:00 AM. I say usually, because I have had
females
broadcast their spawn at 11AM, with all the lights on, as well. During
her broadcasting, she will briskly fan her abdominal pleopods casting
her newly hatched young out into the currents.
When the female senses that the time is near, she will climb to the
highest point of the rock work during the middle of the night, usually
between 1:00 – 3:00 AM. I say usually, because I have had
females
broadcast their spawn at 11AM, with all the lights on, as well. During
her broadcasting, she will briskly fan her abdominal pleopods casting
her newly hatched young out into the currents. Another method used involves moving the female to the larval rearing
kreisel or tank to broadcast her spawn. If the kreisel has a slow flow
the female should not have to be kept contained in any manner within
the kreisel or larval rearing tank as she is quite easy to capture and
move.
Another method used involves moving the female to the larval rearing
kreisel or tank to broadcast her spawn. If the kreisel has a slow flow
the female should not have to be kept contained in any manner within
the kreisel or larval rearing tank as she is quite easy to capture and
move.
 This will take some daily maintenance on your part. Your most immediate
task will involve ensuring that their water quality remains pristine.
The only way you will be able to do so is by performing small water
changes each and every day. Skip a day, and you risk losing your
larvae. Ammonia and nitrites are very deadly to the larvae. These water
changes will also be used to siphon out any and all debris that may
accumulate. I use a foot long piece of rigid tubing that is attached to
a length of airline tubing. The smaller diameter allows greater control
over what you are siphoning out and poses less of a risk of tossing the
baby out with the bathwater, as would happen with larger diameter hoses.
This will take some daily maintenance on your part. Your most immediate
task will involve ensuring that their water quality remains pristine.
The only way you will be able to do so is by performing small water
changes each and every day. Skip a day, and you risk losing your
larvae. Ammonia and nitrites are very deadly to the larvae. These water
changes will also be used to siphon out any and all debris that may
accumulate. I use a foot long piece of rigid tubing that is attached to
a length of airline tubing. The smaller diameter allows greater control
over what you are siphoning out and poses less of a risk of tossing the
baby out with the bathwater, as would happen with larger diameter hoses. After having read a few studies done with the feeding of larval shrimp,
I now only feed my larvae a cyclopoid species of copepods and only
resort to the use of brine shrimp when I am unable to collect copepods
due to the weather. In these studies, it was noted that when the larvae
are fed brine shrimp, the rate of larvae loss is extreme, upwards of 96
percent. This may explain my first poor attempts at keeping the larvae
alive long enough to settle out as I had assumed that baby brine shrimp
would be an adequate food source for the duration of the larvae
development. Harlequin larvae do not swallow food whole, they catch
food and chew on it, so food size is not very critical, although being
too small is no good as the larvae will most likely ignore small food
sources.
After having read a few studies done with the feeding of larval shrimp,
I now only feed my larvae a cyclopoid species of copepods and only
resort to the use of brine shrimp when I am unable to collect copepods
due to the weather. In these studies, it was noted that when the larvae
are fed brine shrimp, the rate of larvae loss is extreme, upwards of 96
percent. This may explain my first poor attempts at keeping the larvae
alive long enough to settle out as I had assumed that baby brine shrimp
would be an adequate food source for the duration of the larvae
development. Harlequin larvae do not swallow food whole, they catch
food and chew on it, so food size is not very critical, although being
too small is no good as the larvae will most likely ignore small food
sources.


 If you have access to a microscope or a quality magnifying glass, you
can remove one of the larvae every few days and watch the changes they
go through after each molt. I find it fascinating to see how after each
molt, they gain another body part or two. Do not be surprised if on
occasion you can not note any changes as shrimp larvae are known to
perform "mark time" molting which appears to be a slowing or delay
mechanism for reasons unknown. Such molting extends the length of time
the larvae remain in a specific stage of their development, which is
why it is impossible to say with certainty how long a given shrimp
species larval period will be. For the harlequin shrimp, the average is
between four to eight weeks.
If you have access to a microscope or a quality magnifying glass, you
can remove one of the larvae every few days and watch the changes they
go through after each molt. I find it fascinating to see how after each
molt, they gain another body part or two. Do not be surprised if on
occasion you can not note any changes as shrimp larvae are known to
perform "mark time" molting which appears to be a slowing or delay
mechanism for reasons unknown. Such molting extends the length of time
the larvae remain in a specific stage of their development, which is
why it is impossible to say with certainty how long a given shrimp
species larval period will be. For the harlequin shrimp, the average is
between four to eight weeks.



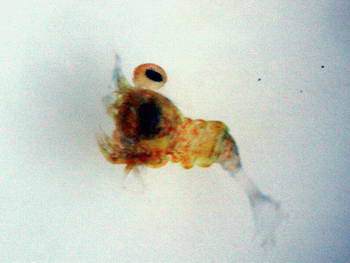
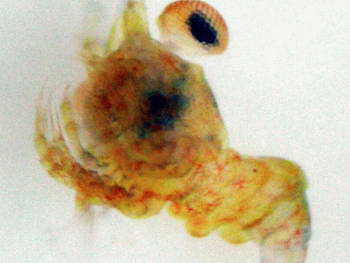
 As you can see, this little endeavor is not for the faint hearted or
those not willing to put in the time each day. Myself, I would, and
hopefully soon, will take satisfaction in not only being
successful with breeding a marine species, but also in being able to
make the young adults available to the trade and thus take that little
bit of pressure off of the wild stocks here locally. Besides that, its
just plain fun! If you have any further questions, you can contact me
through my website and I will do my best to get you the answers to your
questions. Hopefully this article has covered enough ground to answer
the majority of them, or at the least, it got you off on the right
foot. I hope you enjoyed it!
As you can see, this little endeavor is not for the faint hearted or
those not willing to put in the time each day. Myself, I would, and
hopefully soon, will take satisfaction in not only being
successful with breeding a marine species, but also in being able to
make the young adults available to the trade and thus take that little
bit of pressure off of the wild stocks here locally. Besides that, its
just plain fun! If you have any further questions, you can contact me
through my website and I will do my best to get you the answers to your
questions. Hopefully this article has covered enough ground to answer
the majority of them, or at the least, it got you off on the right
foot. I hope you enjoyed it! I would like to thank my wife, Linda, for her continued support and
understanding while I pursue my many marine interests. A special thanks
also goes out to hobby experts, Syd Kraul of Pacific Planktonics, Eric
Borneman and Dr. Ron Shimek for their teachings and advice. I also
highly appreciate the editorial services that Carmalee Scarpitti
contributed. I can only imagine what a herculean effort it must have
been to correct my grammar and spelling, Thank you!
I would like to thank my wife, Linda, for her continued support and
understanding while I pursue my many marine interests. A special thanks
also goes out to hobby experts, Syd Kraul of Pacific Planktonics, Eric
Borneman and Dr. Ron Shimek for their teachings and advice. I also
highly appreciate the editorial services that Carmalee Scarpitti
contributed. I can only imagine what a herculean effort it must have
been to correct my grammar and spelling, Thank you!